Structure and function of the mycobacterial transcription initiation complex with the essential regulator RbpA
- PMID: 28067618
- PMCID: PMC5302886
- DOI: 10.7554/eLife.22520
Structure and function of the mycobacterial transcription initiation complex with the essential regulator RbpA
Abstract
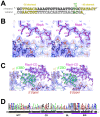
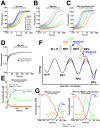
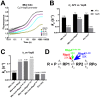
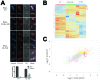
RbpA and CarD are essential transcription regulators in mycobacteria. Mechanistic analyses of promoter open complex (RPo) formation establish that RbpA and CarD cooperatively stimulate formation of an intermediate (RP2) leading to RPo; formation of RP2 is likely a bottleneck step at the majority of mycobacterial promoters. Once RPo forms, CarD also disfavors its isomerization back to RP2. We determined a 2.76 Å-resolution crystal structure of a mycobacterial transcription initiation complex (TIC) with RbpA as well as a CarD/RbpA/TIC model. Both CarD and RbpA bind near the upstream edge of the -10 element where they likely facilitate DNA bending and impede transcription bubble collapse. In vivo studies demonstrate the essential role of RbpA, show the effects of RbpA truncations on transcription and cell physiology, and indicate additional functions for RbpA not evident in vitro. This work provides a framework to understand the control of mycobacterial transcription by RbpA and CarD.
Keywords: E. coli; M. bovis; M. smegmatis; M. tuberculosis; biochemistry; biophysics; structural biology.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures







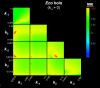
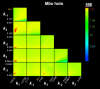
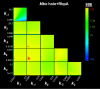
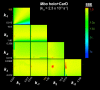
References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D Biological Crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. - DOI - PMC - PubMed
-
- Andrews S. FastQC: a quality control tool for high throughput sequence data. 2010 http://www.bioinformatics.babraham.ac.uk/projects/fastqc
-
- Aramendia PF, Negri RM, Roman ES. Temperature Dependence of Fluorescence and Photoisomerization in symmetric carbocyanines.influence of Medium Viscosity and molecular Structure. The Journal of Physical Chemistry. 1994;98:3165–3173. doi: 10.1021/j100063a020. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

