Focal Adhesion Kinase: Insight into Molecular Roles and Functions in Hepatocellular Carcinoma
- PMID: 28067792
- PMCID: PMC5297733
- DOI: 10.3390/ijms18010099
Focal Adhesion Kinase: Insight into Molecular Roles and Functions in Hepatocellular Carcinoma
Abstract
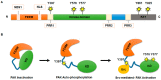
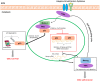
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide. Due to the high incidence of post-operative recurrence after current treatments, the identification of new and more effective drugs is required. In previous years, new targetable genes/pathways involved in HCC pathogenesis have been discovered through the help of high-throughput sequencing technologies. Mutations in TP53 and β-catenin genes are the most frequent aberrations in HCC. However, approaches able to reverse the effect of these mutations might be unpredictable. In fact, if the reactivation of proteins, such as p53 in tumours, holds great promise as anticancer therapy, there are studies arguing that chronic activation of these types of molecules may be deleterious. Thus, recently the efforts on potential targets have focused on actionable mutations, such as those occurring in the gene encoding for focal adhesion kinase (FAK). This tyrosine kinase, localized to cellular focal contacts, is over-expressed in a variety of human tumours, including HCC. Moreover, several lines of evidence demonstrated that FAK depletion or inhibition impair in vitro and in vivo HCC growth and metastasis. Here, we provide an overview of FAK expression and activity in the context of tumour biology, discussing the current evidence of its connection with HCC development and progression.
Keywords: cancer stem cells; focal adhesion kinase; hepatocellular carcinoma; metastasis; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

