Excavatolide B Attenuates Rheumatoid Arthritis through the Inhibition of Osteoclastogenesis
- PMID: 28067799
- PMCID: PMC5295229
- DOI: 10.3390/md15010009
Excavatolide B Attenuates Rheumatoid Arthritis through the Inhibition of Osteoclastogenesis
Abstract
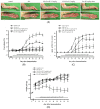
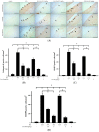
Osteoclasts are multinucleated giant cells of macrophage/monocyte lineage, and cell differentiation with the upregulation of osteoclast-related proteins is believed to play a major role in the destruction of the joints in the course of rheumatoid arthritis (RA). Pro-inflammatory cytokines, such as interleukin-17A (IL-17A) and macrophage colony-stimulating factor (M-CSF), can be overexpressed in RA and lead to osteoclastogenesis. In a previous study, we found that cultured-type soft coral-derived excavatolide B (Exc-B) exhibited anti-inflammatory properties. In the present study, we thus aimed to evaluate the anti-arthritic activity of Exc-B in in vitro and in vivo models. The results demonstrated that Exc-B inhibits LPS-induced multinucleated cell and actin ring formation, as well as TRAP, MMP-9, and cathepsin K expression. Additionally, Exc-B significantly attenuated the characteristics of RA in adjuvant (AIA) and type II collagen-induced arthritis (CIA) in rats. Moreover, Exc-B improved histopathological features, and reduced the number of TRAP-positive multinucleated cells in the in vivo AIA and CIA models. Immunohistochemical analysis showed that Exc-B attenuated the protein expression of cathepsin K, MMP-2, MMP-9, CD11b, and NFATc1 in ankle tissues of AIA and CIA rats. Level of interleukin-17A and macrophage colony-stimulating factor were also decreased by Exc-B. These findings strongly suggest that Exc-B could be of potential use as a therapeutic agent by inhibiting osteoclast differentiation in arthritis. Moreover, this study also illustrates the use of the anti-inflammatory marine compound, Exc-B, as a potential therapeutic strategy for RA.
Keywords: (Exc-B); excavatolide B; osteoclast; osteoclastogenesis; rheumatoid arthritis (RA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

