Gamma aminobutyric acid (GABA) modulators for amyotrophic lateral sclerosis/motor neuron disease
- PMID: 28067943
- PMCID: PMC6953368
- DOI: 10.1002/14651858.CD006049.pub2
Gamma aminobutyric acid (GABA) modulators for amyotrophic lateral sclerosis/motor neuron disease
Abstract
Background: Imbalance of gamma aminobutyric acid (GABA) and related modulators has been implicated as an important factor in the pathogenesis of amyotrophic lateral sclerosis (ALS), which is also known as motor neuron disease (MND). In this context, the role and mechanism of action of gabapentin and baclofen have been extensively investigated, although with conflicting results. This is the first systematic review to assess clinical trials of GABA modulators for the treatment of ALS.
Objectives: To examine the efficacy of gabapentin, baclofen, or other GABA modulators in delaying the progression of ALS, and to evaluate adverse effects of these interventions
Search methods: On 16 August 2016, we searched the Cochrane Neuromuscular Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL Plus, AMED, and LILACS. In addition, we checked the bibliographies of the trials found in order to identify any other trials, and contacted trial authors to identify relevant unpublished results or additional clinical trials. On 30 August 2016, we searched two clinical trials registries.
Selection criteria: Types of studies: double-blind randomized controlled trials (RCTs) or quasi-RCTsTypes of participants: adults with a diagnosis of probable or definite ALSTypes of interventions: gabapentin, baclofen, or other GABA modulators compared with placebo, no treatment, or each otherPrimary outcome: survival at one year from study enrollmentSecondary outcomes: individual rate of decline of maximum voluntary isometric contraction (MVIC), expressed as arm megascore; rate of decline of per cent predicted forced vital capacity (FVC); rate of decline of ALS Functional Rating Scale (ALSFRS); health-related quality of life; survival evaluated by pooling hazards; and adverse events DATA COLLECTION AND ANALYSIS: At least two review authors independently checked titles and abstracts identified by the searches. The review authors obtained and independently analyzed original individual participant data from each included study; additional review authors and the Cochrane Neuromuscular Managing Editor checked the outcome data. Two authors independently assessed the risk of bias in included studies.
Data collection and analysis: At least two review authors independently checked titles and abstracts identified by the searches. The review authors obtained and independently analyzed original individual participant data from each included study; additional review authors and the Cochrane Neuromuscular Managing Editor checked the outcome data. Two authors independently assessed the risk of bias in included studies.
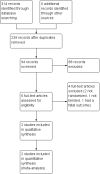
Main results: We identified two double-blind RCTs of gabapentin treatment in ALS for inclusion in this review. We found no eligible RCTs of baclofen or other GABA modulators. The selected studies were phase II and phase III trials, which lasted six and nine months, respectively. They were highly comparable because both were comparisons of oral gabapentin and placebo, performed by the same investigators. The trials enrolled 355 participants with ALS: 80 in the gabapentin group and 72 in the placebo group in the first (phase II) trial and 101 in the gabapentin group and 102 in the placebo group in the second (phase III) trial. Neither trial was long enough to report survival at one year, which was our primary outcome. We found little or no difference in estimated one-year survival between the treated group and the placebo group (78% versus 77%, P = 0.63 by log-rank test; high-quality evidence). We also found little or no difference in the rate of decline of MVIC expressed as arm megascore, or rate of FVC decline (high-quality evidence). One trial investigated monthly decline in the ALSFRS and quality of life measured using the 12-Item Short Form Survey (SF-12) and found little or no difference between groups (moderate-quality evidence). The trials reported similar adverse events. Complaints that were clearly elevated in those taking gabapentin, based on analyses of the combined data, were light-headedness, drowsiness, and limb swelling (high-quality evidence). Fatigue and falls occurred more frequently with gabapentin than with placebo in one trial, but when we combined the data for fatigue from both trials, there was no clear difference between the groups. We assessed the overall risk of bias in the included trials as low.
Authors' conclusions: According to high-quality evidence, gabapentin is not effective in treating ALS. It does not extend survival, slow the rate of decline of muscle strength, respiratory function and, based on moderate-quality evidence, probably does not improve quality of life or slow monthly decline in the ALSFRS. Other GABA modulators have not been studied in randomized trials.
Conflict of interest statement
Andrea Diana: none known.
Rita Pillai: none known.
Paolo Bongioanni: none known.
Aidan G O'Keeffe: none known.
Robert G Miller: conducted two of the clinical trials of gabapentin (Miller 1996; Miller 2001). He also served on the advisory board of the ALS CARE program, which was the recipient of an unrestricted grant from the manufacturer of the drug riluzole.
Dan H Moore: conducted two of the clinical trials of gabapentin (Miller 1996; Miller 2001). He also served on the advisory board of the ALS CARE program, which was the recipient of an unrestricted grant from the manufacturer of the drug riluzole.
Figures


Update of
References
References to studies included in this review
Miller 1996 {published data only}
-
- Miller RG, Moore D, Young LA, Armon C, Barohn RJ, Bromberg MB, et al. Placebo‐controlled trial of gabapentin in patients with amyotrophic lateral sclerosis. WALS Study Group. Western Amyotrophic Lateral Sclerosis Study Group. Neurology 1996;47(6):1383‐8. [PUBMED: 8960715] - PubMed
Miller 2001 {published data only}
-
- Miller RG, Moore DH 2nd, Gelinas DF, Dronsky V, Mendoza M, Barohn RJ, et al. Phase III randomized trial of gabapentin in patients with amyotrophic lateral sclerosis. Neurology 2001;56(7):843‐8. [PUBMED: 11294919] - PubMed
References to studies excluded from this review
Caramia 2000 {published data only}
-
- Caramia MD, Palmieri MG, Desiato MT, Iani C, Scalise A, Telera S, et al. Pharmacologic reversal of cortical hyperexcitability in patients with ALS. Neurology 2000;54(1):58‐64. [PUBMED: 10636126] - PubMed
Kalra 2003 {published data only}
Mazzini 1998 {published data only}
-
- Mazzini L, Mora G, Balzarini C, Brigatti M, Pirali I, Comazzi F, et al. The natural history and the effects of gabapentin in amyotrophic lateral sclerosis. Journal of the Neurological Sciences 1998;160(Suppl 1):S57‐63. [PUBMED: 9851651] - PubMed
Norris 1979 {published data only}
-
- Norris FH Jr, Kwei Sang U, Sachais B, Carey M. Trial of baclofen in amyotrophic lateral sclerosis. Archives of Neurology 1979;36(11):715‐6. [PUBMED: 508132] - PubMed
Additional references
Ashworth 2012
-
- Ashworth NL, Satkunam LE, Deforge D. Treatment for spasticity in amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD004156.pub4] - DOI
Baldinger 2012
Brooks 2000
-
- Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders 2000;1(5):293‐9. - PubMed
Bryans 1999
-
- Bryans JS, Horwell DC, Ratcliffe GS, Receveur JM, Rubin JR. An in vitro investigation into conformational aspects of gabapentin. Bioorganic and Medicinal Chemistry 1999;7(5):715‐21. - PubMed
Cedarbaum 1999
-
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS‐R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). Journal of Neurological Sciences 1999;169(1‐2):13‐21. - PubMed
Chiò 2013
Diana 2006
GRADE 2008 [Computer program]
-
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kostera 2002
-
- Kostera‐Pruszczyk A, Niebroj‐Dobosz I, Emeryk‐Szajewska B, Karwańska A, Rowińska‐Marcińska K. Motor unit hyperexcitability in amyotrophic lateral sclerosis vs amino acids acting as neurotransmitters. Acta Neurologica Scandinavica 2002;106(1):34‐8. - PubMed
Lloyd 2000
-
- Lloyd CM, Richardson MP, Brooks DJ, Al‐Chalabi A, Leigh PN. Extramotor involvement in ALS: PET studies with GABA(A) ligand [(11)C]flumazenil. Brain 2000;123(11):2289‐96. - PubMed
Niebroj‐Dobosz 1999
-
- Niebroj‐Dobosz I, Janik P. Amino acids acting as transmitters in amyotrophic lateral sclerosis (ALS). Acta Neurologica Scandinavica 1999;100(1):6‐11. - PubMed
Parker 2004
-
- Parker DA, Ong J, Marino V, Kerr DI. Gabapentin activates presynaptic GABAB heteroreceptors in rat cortical slices. European Journal of Pharmacology 2004;495(2‐3):137‐43. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. Erratum in: Statistics in Medicine 2004; 23(11):1817. - PubMed
Petri 2003
-
- Petri S, Krampfl K, Hashemi F, Grothe C, Hori A, Dengler R, et al. Distribution of GABAA receptor mRNA in the motor cortex of ALS patients. Journal of Neuropathology and Experimental Neurology 2003;62(10):1041‐51. - PubMed
Plaitakis 1987
-
- Plaitakis A, Caroscio JT. Abnormal glutamate metabolism in amyotrophic lateral sclerosis. Annals of Neurology 1987;22(5):575‐9. - PubMed
Rand 2016
-
- Rand Corporation. 36‐Item Short Form Survey (SF‐36). www.rand.org/health/surveys_tools/mos/36‐item‐short‐form.html (accessed prior to 26 November 2016).
Rao 2004
-
- Rao SD, Weiss JH. Excitotoxic and oxidative cross‐talk between motor neurons and glia in ALS pathogenesis. Trends in Neurosciences 2004;27(1):17‐23. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rothstein 1991
-
- Rothstein JD, Kuncl R, Chaudhry V, Clawson L, Cornblath DR, Coyle JT, et al. Excitatory amino acids in amyotrophic lateral sclerosis: an update. Annals of Neurology 1991;30(2):224‐5. - PubMed
Satzinger 1994
-
- Satzinger G. Antiepileptics from gamma‐aminobutyric acid. Arzneimittel‐Forschung 1994;44(3):261‐6. - PubMed
Shaw 1995
-
- Shaw PJ, Forrest V, Ince PG, Richardson JP, Wastell HJ. CSF and plasma amino acid levels in motor neuron disease: elevation of CSF glutamate in a subset of patients. Neurodegeneration 1995;4(2):209‐16. - PubMed
Spreux‐Varoquaux 2002
-
- Spreux‐Varoquaux O, Bensimon G, Lacomblez L, Salachas F, Pradat PF, Forestier N, et al. Glutamate levels in cerebrospinal fluid in amyotrophic lateral sclerosis: a reappraisal using a new HPLC method with coulometric detection in a large cohort of patients. Journal of the Neurological Sciences 2002;193(2):73‐8. - PubMed
Stata 2009 [Computer program]
-
- StataCorp. Stata Statistical Software. Version 11. College Station, TX: StataCorp LP, 2009.
Taylor 1994
-
- Taylor CP. Emerging perspectives on the mechanism of action of gabapentin. Neurology 1994;44(6 Suppl 5):S10‐6; discussion S31‐2. - PubMed
Taylor 1998
-
- Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Research 1998;29(3):233‐49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

