Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics
- PMID: 28068069
- PMCID: PMC5604727
- DOI: 10.1021/acsnano.6b07607
Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics
Abstract
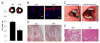
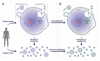
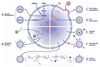
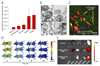
In the past decade, extracellular vesicles (EVs) have emerged as a key cell-free strategy for the treatment of a range of pathologies, including cancer, myocardial infarction, and inflammatory diseases. Indeed, the field is rapidly transitioning from promising in vitro reports toward in vivo animal models and early clinical studies. These investigations exploit the high physicochemical stability and biocompatibility of EVs as well as their innate capacity to communicate with cells via signal transduction and membrane fusion. This review focuses on methods in which EVs can be chemically or biologically modified to broaden, alter, or enhance their therapeutic capability. We examine two broad strategies, which have been used to introduce a wide range of nanoparticles, reporter systems, targeting peptides, pharmaceutics, and functional RNA molecules. First, we explore how EVs can be modified by manipulating their parent cells, either through genetic or metabolic engineering or by introducing exogenous material that is subsequently incorporated into secreted EVs. Second, we consider how EVs can be directly functionalized using strategies such as hydrophobic insertion, covalent surface chemistry, and membrane permeabilization. We discuss the historical context of each specific technology, present prominent examples, and evaluate the complexities, potential pitfalls, and opportunities presented by different re-engineering strategies.
Keywords: cell-free therapy; drug loading; exosomes; extracellular vesicles; functionalization; genetic manipulation; membrane modification; microvesicles.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

