Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice
- PMID: 28068222
- PMCID: PMC5668137
- DOI: 10.1016/j.cmet.2016.09.013
Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice
Abstract
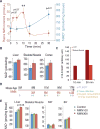
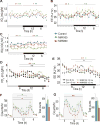
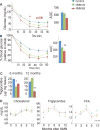
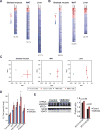
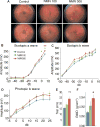
NAD+ availability decreases with age and in certain disease conditions. Nicotinamide mononucleotide (NMN), a key NAD+ intermediate, has been shown to enhance NAD+ biosynthesis and ameliorate various pathologies in mouse disease models. In this study, we conducted a 12-month-long NMN administration to regular chow-fed wild-type C57BL/6N mice during their normal aging. Orally administered NMN was quickly utilized to synthesize NAD+ in tissues. Remarkably, NMN effectively mitigates age-associated physiological decline in mice. Without any obvious toxicity or deleterious effects, NMN suppressed age-associated body weight gain, enhanced energy metabolism, promoted physical activity, improved insulin sensitivity and plasma lipid profile, and ameliorated eye function and other pathophysiologies. Consistent with these phenotypes, NMN prevented age-associated gene expression changes in key metabolic organs and enhanced mitochondrial oxidative metabolism and mitonuclear protein imbalance in skeletal muscle. These effects of NMN highlight the preventive and therapeutic potential of NAD+ intermediates as effective anti-aging interventions in humans.
Keywords: NAD(+); NAD(+) precursor; NMN; aging; anti-aging; energy metabolism; eye function; glucose metabolism; insulin sensitivity; mitochondria; nicotinamide mononucleotide.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
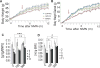
References
-
- Anderson R, Bitterman K, Wood J, Medvedik O, Cohen H, Lin S, Manchester J, Gordon J, Sinclair D. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J. Biol. Chem. 2002;277:18881–18890. - PubMed
-
- Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+ Cell. 2007;129:473–484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

