Role of Akt2 in regulation of metastasis suppressor 1 expression and colorectal cancer metastasis
- PMID: 28068324
- PMCID: PMC5667907
- DOI: 10.1038/onc.2016.460
Role of Akt2 in regulation of metastasis suppressor 1 expression and colorectal cancer metastasis
Abstract
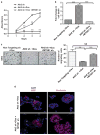
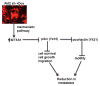
Survival signaling is critical for the metastatic program of cancer cells. The current study investigated the role of Akt survival proteins in colorectal cancer (CRC) metastasis and explored potential mechanisms of Akt-mediated metastasis regulation. Using an orthotopic implantation model in mice, which uniquely recapitulates the entire multistep process of CRC metastasis, combined with an inducible system of short hairpin RNA-mediated Akt isoform knockdown in human CRC cells, our studies confirm a role of Akt2 in CRC cell dissemination to distant organs in vivo. Akt2 deficiency profoundly inhibited the development of liver lesions in mice, whereas Akt1 had no effect under the experimental conditions used in the study. Array analysis of human metastatic genes identified the scaffolding protein metastasis suppressor 1 (MTSS1) as a novel Akt2-regulated gene. Inducible loss of Akt2 in CRC cells robustly upregulated MTSS1 at the messenger RNA and protein level, and the accumulated protein was functionally active as shown by its ability to engage an MTSS1-Src-cortactin inhibitory axis. MTSS1 expression led to a marked reduction in levels of functional cortacin (pcortactin Y421), an actin nucleation-promoting factor that has a crucial role in cancer cell invasion and metastasis. MTSS1 was also shown to mediate suppressive effects of Akt2 deficiency on CRC cell viability, survival, migration and actin polymerization in vitro. The relevance of these findings to human CRC is supported by analysis of The Cancer Genome Atlas (TCGA) and NCBI GEO data sets, which demonstrated inverse changes in expression of Akt2 and MTSS1 during CRC progression. Taken together, the data identify MTSS1 as a new Akt2-regulated gene, and point to suppression of MTSS1 as a key step in the metastasis-promoting effects of Akt2 in CRC cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




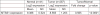
Similar articles
-
MTSS1 inhibits colorectal cancer metastasis by regulating the CXCR4/CXCL12 signaling axis.Int J Mol Med. 2021 May;47(5):65. doi: 10.3892/ijmm.2021.4898. Epub 2021 Mar 2. Int J Mol Med. 2021. Retraction in: Int J Mol Med. 2023 Feb;51(2):15. doi: 10.3892/ijmm.2023.5218. PMID: 33649808 Free PMC article. Retracted.
-
MicroRNA-135b regulates metastasis suppressor 1 expression and promotes migration and invasion in colorectal cancer.Mol Cell Biochem. 2014 Mar;388(1-2):249-59. doi: 10.1007/s11010-013-1916-z. Epub 2013 Dec 17. Mol Cell Biochem. 2014. PMID: 24343340
-
Inhibition of miR-15b decreases cell migration and metastasis in colorectal cancer.Tumour Biol. 2016 Jul;37(7):8765-73. doi: 10.1007/s13277-015-4396-9. Epub 2016 Jan 7. Tumour Biol. 2016. PMID: 26743779
-
MTSS1: a multifunctional protein and its role in cancer invasion and metastasis.Front Biosci (Schol Ed). 2011 Jan 1;3(2):621-31. doi: 10.2741/s175. Front Biosci (Schol Ed). 2011. PMID: 21196400 Review.
-
Triangle of AKT2, miRNA, and Tumorigenesis in Different Cancers.Appl Biochem Biotechnol. 2018 Jun;185(2):524-540. doi: 10.1007/s12010-017-2657-3. Epub 2017 Dec 4. Appl Biochem Biotechnol. 2018. PMID: 29199386 Review.
Cited by
-
UEG Week 2020 Poster Presentations.United European Gastroenterol J. 2020 Oct;8(8_suppl):144-887. doi: 10.1177/2050640620927345. United European Gastroenterol J. 2020. PMID: 33043826 Free PMC article. No abstract available.
-
AKT3 Expression in Mesenchymal Colorectal Cancer Cells Drives Growth and Is Associated with Epithelial-Mesenchymal Transition.Cancers (Basel). 2021 Feb 14;13(4):801. doi: 10.3390/cancers13040801. Cancers (Basel). 2021. PMID: 33673003 Free PMC article.
-
AKT isoform-specific expression and activation across cancer lineages.BMC Cancer. 2018 Jul 16;18(1):742. doi: 10.1186/s12885-018-4654-5. BMC Cancer. 2018. PMID: 30012111 Free PMC article.
-
The transcribed pseudogene RPSAP52 enhances the oncofetal HMGA2-IGF2BP2-RAS axis through LIN28B-dependent and independent let-7 inhibition.Nat Commun. 2019 Sep 4;10(1):3979. doi: 10.1038/s41467-019-11910-6. Nat Commun. 2019. PMID: 31484926 Free PMC article.
-
Contributions of Thyroid Hormone to Cancer Metastasis.Biomedicines. 2018 Aug 22;6(3):89. doi: 10.3390/biomedicines6030089. Biomedicines. 2018. PMID: 30135398 Free PMC article. Review.
References
-
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- National Cancer Institute. SEER Stat Fact Sheets: Colon and Rectum Cancer. Surveillance, Epidemiology and End Results Program [online] 2010
-
- Weigelt B, Peterse JL, van 't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. - PubMed
-
- Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2009;16:116–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

