Microbiologically Confirmed Tuberculosis: Factors Associated with Pre-Treatment Loss to Follow-Up, and Time to Treatment Initiation
- PMID: 28068347
- PMCID: PMC5222612
- DOI: 10.1371/journal.pone.0168659
Microbiologically Confirmed Tuberculosis: Factors Associated with Pre-Treatment Loss to Follow-Up, and Time to Treatment Initiation
Abstract
Background: The impact of new diagnostics on pre-treatment loss to follow up (Pre-treatment LTFU) has not been widely investigated. The reported rate of pre-treatment LTFU is however lower in studies where Xpert MTB/Rif (Xpert) has been used onsite as opposed to centrally. The use of the Xpert at point of care (POC) could have a role in reducing the pre-treatment LTFU rate among TB patients. We aimed to determine the pre-treatment LTFU rate and the time to treatment initiation as well as to describe associated factors in patients diagnosed with TB using POC Xpert or smear microscopy.
Method: Xpert machines were installed at 7 primary healthcare facilities in inner-city Johannesburg. POC Xpert TB testing was the primary diagnostic method for all patients although there were some patients who were tested using only laboratory-based smear microscopy (during power outages or machine operator off-sick). Data on patients' demographics, TB diagnostic test (Xpert or smear microscopy), test result, and time to treatment initiation were collected. Associations and predictors of pre-treatment LTFU and time to treatment initiation were explored.
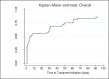
Findings: A total of 1981 people with presumptive TB were tested (1743 using Xpert and 238 using smear). A bacteriological diagnosis of TB was made in 271 patients (90% Xpert; 10% smear). The median time to treatment initiation in the smear group was 9 days (IQR: 4-20) while those tested using Xpert had a median time of 0 days (IQR: 0-0). Pre-treatment LTFU was 22.5% with no difference between diagnostic groups (p = 0.8).
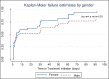
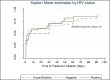
Conclusion: The Pre-treatment LTFU rate of 22.5% found in this study is much higher than the 5% target of the South African National TB Control Program. POC Xpert resulted in a significantly greater proportion of bacteriologically proven TB patients being started on treatment within 30 days of presentation. No risk factors associated with pre-treatment LTFU were identified.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Organization WH. Global TB Report. 2015.
-
- Cox HS, Mbhele S, Mohess N, Whitelaw A, Muller O, Zemanay W, et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLoS medicine. 2014;11(11):e1001760 Epub 2014/11/26. PubMed Central PMCID: PMC4244039. 10.1371/journal.pmed.1001760 - DOI - PMC - PubMed
-
- Durovni B, Saraceni V, van den Hof S, Trajman A, Cordeiro-Santos M, Cavalcante S, et al. Impact of replacing smear microscopy with Xpert MTB/RIF for diagnosing tuberculosis in Brazil: a stepped-wedge cluster-randomized trial. PLoS medicine. 2014;11(12):e1001766 Epub 2014/12/10. PubMed Central PMCID: PMC4260794. 10.1371/journal.pmed.1001766 - DOI - PMC - PubMed
-
- Churchyard GJ, Stevens WS, Mametja LD, McCarthy KM, Chihota V, Nicol MP, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. The Lancet Global health. 2015;3(8):e450–7. Epub 2015/07/19. 10.1016/S2214-109X(15)00100-X - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

