Immunity to Commensal Fungi: Detente and Disease
- PMID: 28068483
- PMCID: PMC6573037
- DOI: 10.1146/annurev-pathol-052016-100342
Immunity to Commensal Fungi: Detente and Disease
Abstract
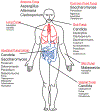
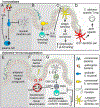
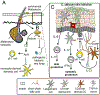
Fungi are ubiquitous in our environment, and a healthy immune system is essential to maintain adequate protection from fungal infections. When this protection breaks down, superficial and invasive fungal infections cause diseases that range from irritating to life-threatening. Millions of people worldwide develop invasive infections during their lives, and mortality for these infections often exceeds 50%. Nevertheless, we are normally colonized with many of the same disease-causing fungi (e.g., on the skin or in the gut). Recent research is dramatically expanding our understanding of the mechanisms by which our immune systems interact with these organisms in health and disease. In this review, we discuss what is currently known about where and how the immune system interacts with common fungi.
Keywords: Aspergillus; Candida; Dectin-1; Malassezia; innate immunity; microbiome; mycobiome.
Figures
References
-
- Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. 2015. Immune defence against Candida fungal infections. Nat. Rev. Immunol 15: 630–42 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

