Kinetically E-selective macrocyclic ring-closing metathesis
- PMID: 28068669
- PMCID: PMC5247355
- DOI: 10.1038/nature20800
Kinetically E-selective macrocyclic ring-closing metathesis
Abstract
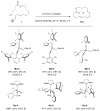
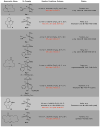
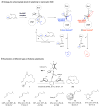
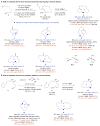
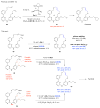
Macrocyclic compounds are central to the development of new drugs, but preparing them can be challenging because of the energy barrier that must be surmounted in order to bring together and fuse the two ends of an acyclic precursor such as an alkene (also known as an olefin). To this end, the catalytic process known as ring-closing metathesis (RCM) has allowed access to countless biologically active macrocyclic organic molecules, even for large-scale production. Stereoselectivity is often critical in such cases: the potency of a macrocyclic compound can depend on the stereochemistry of its alkene; alternatively, one isomer of the compound can be subjected to stereoselective modification (such as dihydroxylation). Kinetically controlled Z-selective RCM reactions have been reported, but the only available metathesis approach for accessing macrocyclic E-olefins entails selective removal of the Z-component of a stereoisomeric mixture by ethenolysis, sacrificing substantial quantities of material if E/Z ratios are near unity. Use of ethylene can also cause adventitious olefin isomerization-a particularly serious problem when the E-alkene is energetically less favoured. Here, we show that dienes containing an E-alkenyl-B(pinacolato) group, widely used in catalytic cross-coupling, possess the requisite electronic and steric attributes to allow them to be converted stereoselectively to E-macrocyclic alkenes. The reaction is promoted by a molybdenum monoaryloxide pyrrolide complex and affords products at a yield of up to 73 per cent and an E/Z ratio greater than 98/2. We highlight the utility of the approach by preparing recifeiolide (a 12-membered-ring antibiotic) and pacritinib (an 18-membered-ring enzyme inhibitor), the Z-isomer of which is less potent than the E-isomer. Notably, the 18-membered-ring moiety of pacritinib-a potent anti-cancer agent that is in advanced clinical trials for treating lymphoma and myelofibrosis-was prepared by RCM carried out at a substrate concentration 20 times greater than when a ruthenium carbene was used.
Conflict of interest statement
The authors declare competing financial interests: AHH and RRS are cofounders of a company that has licensed the technology reported in this manuscript.
Figures


Similar articles
-
Efficient and selective formation of macrocyclic disubstituted Z alkenes by ring-closing metathesis (RCM) reactions catalyzed by Mo- or W-based monoaryloxide pyrrolide (MAP) complexes: applications to total syntheses of epilachnene, yuzu lactone, ambrettolide, epothilone C, and nakadomarin A.Chemistry. 2013 Feb 18;19(8):2726-40. doi: 10.1002/chem.201204045. Epub 2013 Jan 23. Chemistry. 2013. PMID: 23345004 Free PMC article.
-
Stereoselective access to Z and E macrocycles by ruthenium-catalyzed Z-selective ring-closing metathesis and ethenolysis.J Am Chem Soc. 2013 Jan 9;135(1):94-7. doi: 10.1021/ja311241q. Epub 2012 Dec 21. J Am Chem Soc. 2013. PMID: 23244210 Free PMC article.
-
Stereoselectivity of macrocyclic ring-closing olefin metathesis.Org Lett. 2000 Jul 13;2(14):2145-7. doi: 10.1021/ol006059s. Org Lett. 2000. PMID: 10891252
-
Recent applications of olefin ring-closing metathesis (RCM) in the synthesis of biologically important alkaloids, terpenoids, polyketides and other secondary metabolites.Curr Top Med Chem. 2005;5(15):1473-94. doi: 10.2174/156802605775009793. Curr Top Med Chem. 2005. PMID: 16378488 Review.
-
The application of olefin metathesis to the synthesis of biologically active macrocyclic agents.Curr Top Med Chem. 2005;5(15):1495-519. doi: 10.2174/156802605775009748. Curr Top Med Chem. 2005. PMID: 16378489 Review.
Cited by
-
E- and Z-trisubstituted macrocyclic alkenes for natural product synthesis and skeletal editing.Nat Chem. 2022 Jun;14(6):640-649. doi: 10.1038/s41557-022-00935-y. Epub 2022 May 16. Nat Chem. 2022. PMID: 35577918 Free PMC article.
-
Molybdenum(VI) Bis(imido) Complexes: From Frustrated Lewis Pairs to Weakly Coordinating Cations.Chemistry. 2022 Oct 4;28(55):e202201867. doi: 10.1002/chem.202201867. Epub 2022 Aug 3. Chemistry. 2022. PMID: 35775999 Free PMC article.
-
Air-stable 18-electron adducts of Schrock catalysts with tuned stability constants for spontaneous release of the active species.Commun Chem. 2021 May 19;4(1):71. doi: 10.1038/s42004-021-00503-4. Commun Chem. 2021. PMID: 36697610 Free PMC article.
-
Synthesis of 2,6-Hexa-tert-butylterphenyl Derivatives, 2,6-(2,4,6-t-Bu3C6H2)2C6H3X, where X = I, Li, OH, SH, N3, or NH2.Org Lett. 2017 May 19;19(10):2607-2609. doi: 10.1021/acs.orglett.7b01062. Epub 2017 May 1. Org Lett. 2017. PMID: 28459588 Free PMC article.
-
Inhibitors of protein-protein interactions (PPIs): an analysis of scaffold choices and buried surface area.Curr Opin Chem Biol. 2018 Jun;44:75-86. doi: 10.1016/j.cbpa.2018.06.004. Epub 2018 Jun 13. Curr Opin Chem Biol. 2018. PMID: 29908451 Free PMC article. Review.
References
-
- Martí-Centelles V, Pandey MD, Burguete MI, Luis SV. Macrocyclization reactions: The importance of conformational, configurational, and template-induced preorganization. Chem Rev. 2015;115:8736–8834. - PubMed
-
- Hanson PR, Maitram S, Chegondi R, Markley JL. In: Handbook of Metathesis. Grubbs RH, O’Leary DJ, editors. Vol. 2. Wiley–VCH; 2014. pp. 1–170.
-
- Mallinson J, Collins I. Macrocycles in new drug discovery. Future Med Chem. 2012;4:1409–1438. - PubMed
-
- Gradillas A, Perez-Castells J. In: Metathesis in Natural Product Synthesis. Cossy J, Arseniyades S, Meyer C, editors. Wiley–VCH; 2010. pp. 149–182.
-
- Higman CS, Lummiss JAM, Fogg DE. Olefin metathesis at the dawn of implementation in pharmaceutical and specialty-chemicals manufacturing. Angew Chem Int Ed. 2016;55:3552–3565. - PubMed
Additional References
-
- Brown HC, Gupta SK. Hydroboration. XXXIX 1,3,2-Benzodioxaborole (catecholborane) as a new hydroboration reagent for alkenes and alkynes General synthesis of alkane- and alkeneboronic acids and esters via hydroboration Directive effects in the hydroboration of alkenes and alkynes with catecholborane. J Am Chem Soc. 1975;97:5249–5255.
-
- Tucker CE, Davidson J, Knochel P. Mild and stereoselective hydroborations of functionalized alkynes and alkenes using pinacolborane. J Org Chem. 1992;57:3482–3485.
-
- Takai K, Shinomiya N, Kaihara H, Yoshida N, Moriwake T, Utimoto K. Transformation of aldehydes into (E)-1-alkenylboronic esters with a geminal dichromium reagent derived from a dichloromethylboronic ester and CrCl2. Synlett. 1995;9:963–964.
-
- Pereira S, Srebnik M. Hydroboration of alkynes with pinacolborane catalyzed by HZrCp2Cl. Organometallics. 1995;14:3127–3128.
-
- Morrill C, Grubbs RH. Synthesis of functionalized vinyl boronates via ruthenium-catalyzed olefin cross-metathesis and subsequent conversion to vinyl halides. J Org Chem. 2003;68:6031–6034. - PubMed

