Long-Acting Injectable Naltrexone Induction: A Randomized Trial of Outpatient Opioid Detoxification With Naltrexone Versus Buprenorphine
- PMID: 28068780
- PMCID: PMC5411308
- DOI: 10.1176/appi.ajp.2016.16050548
Long-Acting Injectable Naltrexone Induction: A Randomized Trial of Outpatient Opioid Detoxification With Naltrexone Versus Buprenorphine
Abstract
Objective: At present there is no established optimal approach for transitioning opioid-dependent adults to extended-release injection naltrexone (XR-naltrexone) while preventing relapse. The authors conducted a trial examining the efficacy of two methods of outpatient opioid detoxification for induction to XR-naltrexone.
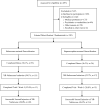
Method: Participants were 150 opioid-dependent adults randomly assigned 2:1 to one of two outpatient detoxification regimens, naltrexone-assisted detoxification or buprenorphine-assisted detoxification, followed by an injection of XR-naltrexone. Naltrexone-assisted detoxification lasted 7 days and included a single day of buprenorphine followed by ascending doses of oral naltrexone along with clonidine and other adjunctive medications. Buprenorphine-assisted detoxification included a 7-day buprenorphine taper followed by a week-long delay before administration of XR-naltrexone, consistent with official prescribing information for XR-naltrexone. Participants from both groups received behavioral therapy focused on medication adherence and a second dose of XR-naltrexone.
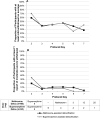
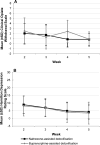
Results: Compared with participants in the buprenorphine-assisted detoxification condition, participants assigned to naltrexone-assisted detoxification were significantly more likely to be successfully inducted to XR-naltrexone (56.1% compared with 32.7%) and to receive the second injection at week 5 (50.0% compared with 26.9%). Both models adjusted for primary type of opioid use, route of opioid administration, and morphine equivalents at baseline.
Conclusions: These results demonstrate the safety, efficacy, and tolerability of low-dose naltrexone, in conjunction with single-day buprenorphine dosing and adjunctive nonopioid medications, for initiating adults with opioid dependence to XR-naltrexone. This strategy offers a promising alternative to the high rates of attrition and relapse currently observed with agonist tapers in both inpatient and outpatient settings.
Trial registration: ClinicalTrials.gov NCT01377610.
Keywords: Buprenorphine; Detoxification; Injectable Naltrexone; Naltrexone; Opioid Dependence; Outpatient.
Conflict of interest statement
All remaining authors report no financial relationships with commercial interests.
Figures
Comment in
-
Current Progress in Opioid Treatment.Am J Psychiatry. 2017 May 1;174(5):414-416. doi: 10.1176/appi.ajp.2016.16121398. Am J Psychiatry. 2017. PMID: 28457152 No abstract available.
References
-
- Center for Behavioral Health Statistics and Quality. (HHS Publication No. SMA 15-4927, NSDUH Series H-50).Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015 Retrieved from http://www.samhsa.gov/data/. Accessed on April 24, 2016.
-
- Centers for Disease Control and Prevention (CDC) CDC – Prescription Painkiller Overdoses Policy Impact Brief – Home and Recreational Safety. 2013
-
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths – United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 64(50–51):1378–82. - PubMed
-
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled multicentre randomised trial. Lancet. 2011;377(9776):1506–13. - PubMed
-
- Physicians Desk Reference. 2015 http://www.pdr.net/drug-summary/vivitrol?druglabelid=1199&id=2886.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

