CHERISH (collaboration for hospitalised elders reducing the impact of stays in hospital): protocol for a multi-site improvement program to reduce geriatric syndromes in older inpatients
- PMID: 28068906
- PMCID: PMC5223473
- DOI: 10.1186/s12877-016-0399-7
CHERISH (collaboration for hospitalised elders reducing the impact of stays in hospital): protocol for a multi-site improvement program to reduce geriatric syndromes in older inpatients
Abstract
Background: Older inpatients are at risk of hospital-associated geriatric syndromes including delirium, functional decline, incontinence, falls and pressure injuries. These contribute to longer hospital stays, loss of independence, and death. Effective interventions to reduce geriatric syndromes remain poorly implemented due to their complexity, and require an organised approach to change care practices and systems. Eat Walk Engage is a complex multi-component intervention with structured implementation, which has shown reduced geriatric syndromes and length of stay in pilot studies at one hospital. This study will test effectiveness of implementing Eat Walk Engage using a multi-site cluster randomised trial to inform transferability of this intervention.
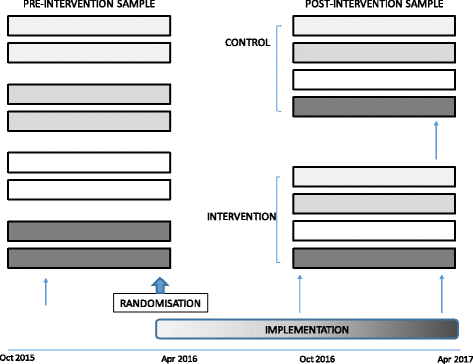
Methods: A hybrid study design will evaluate the effectiveness and implementation strategy of Eat Walk Engage in a real-world setting. A multisite cluster randomised study will be conducted in 8 medical and surgical wards in 4 hospitals, with one ward in each site randomised to implement Eat Walk Engage (intervention) and one to continue usual care (control). Intervention wards will be supported to develop and implement locally tailored strategies to enhance early mobility, nutrition, and meaningful activities. Resources will include a trained, mentored facilitator, audit support, a trained healthcare assistant, and support by an expert facilitator team using the i-PARIHS implementation framework. Patient outcomes and process measures before and after intervention will be compared between intervention and control wards. Primary outcomes are any hospital-associated geriatric syndrome (delirium, functional decline, falls, pressure injuries, new incontinence) and length of stay. Secondary outcomes include discharge destination; 30-day mortality, function and quality of life; 6 month readmissions; and cost-effectiveness. Process measures including patient interviews, activity mapping and mealtime audits will inform interventions in each site and measure improvement progress. Factors influencing the trajectory of implementation success will be monitored on implementation wards.
Discussion: Using a hybrid design and guided by an explicit implementation framework, the CHERISH study will establish the effectiveness, cost-effectiveness and transferability of a successful pilot program for improving care of older inpatients, and identify features that support successful implementation.
Trial registration: ACTRN12615000879561 registered prospectively 21/8/2015.
Keywords: Acute care; Aged; Delirium; Elderly; Facilitation; Falls; Functional decline; Hospital care organisation; Hospitalisation; Implementation; Implementation framework; Incontinence; Pressure injuries; Pressure ulcer; Urinary incontinence.
Figures
References
-
- AIHW . Australian hospital statistics 2011–12. In: AIHW, editor. Health services series 50. Canberra: AIHW; 2013.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

