High dietary folate in pregnant mice leads to pseudo-MTHFR deficiency and altered methyl metabolism, with embryonic growth delay and short-term memory impairment in offspring
- PMID: 28069796
- PMCID: PMC5409086
- DOI: 10.1093/hmg/ddx004
High dietary folate in pregnant mice leads to pseudo-MTHFR deficiency and altered methyl metabolism, with embryonic growth delay and short-term memory impairment in offspring
Abstract
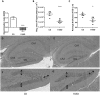
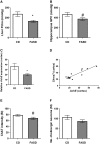
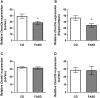
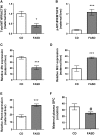
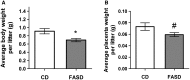
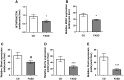
Methylenetetrahydrofolate reductase (MTHFR) generates methyltetrahydrofolate for methylation reactions. Severe MTHFR deficiency results in homocystinuria and neurologic impairment. Mild MTHFR deficiency (677C > T polymorphism) increases risk for complex traits, including neuropsychiatric disorders. Although low dietary folate impacts brain development, recent concerns have focused on high folate intake following food fortification and increased vitamin use. Our goal was to determine whether high dietary folate during pregnancy affects brain development in murine offspring. Female mice were placed on control diet (CD) or folic acid-supplemented diet (FASD) throughout mating, pregnancy and lactation. Three-week-old male pups were evaluated for motor and cognitive function. Tissues from E17.5 embryos, pups and dams were collected for choline/methyl metabolite measurements, immunoblotting or gene expression of relevant enzymes. Brains were examined for morphology of hippocampus and cortex. Pups of FASD mothers displayed short-term memory impairment, decreased hippocampal size and decreased thickness of the dentate gyrus. MTHFR protein levels were reduced in FASD pup livers, with lower concentrations of phosphocholine and glycerophosphocholine in liver and hippocampus, respectively. FASD pup brains showed evidence of altered acetylcholine availability and Dnmt3a mRNA was reduced in cortex and hippocampus. E17.5 embryos and placentas from FASD dams were smaller. MTHFR protein and mRNA were reduced in embryonic liver, with lower concentrations of choline, betaine and phosphocholine. Embryonic brain displayed altered development of cortical layers. In summary, high folate intake during pregnancy leads to pseudo-MTHFR deficiency, disturbed choline/methyl metabolism, embryonic growth delay and memory impairment in offspring. These findings highlight the unintended negative consequences of supplemental folic acid.
© The Author 2017. Published by Oxford University Press.
Figures
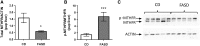
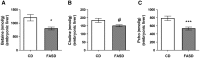

References
-
- Bottiglieri T. (1996) Folate, vitamin B12, and neuropsychiatric disorders. Nutr. Rev., 54, 382–390. - PubMed
-
- Frosst P., Blom H.J., Milos R., Goyette P., Sheppard C.A., Matthews R.G., Boers G.J., den Heijer M., Kluijtmans L.A., van den Heuvel L.P., et al. (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat. Genet., 10, 111–113. - PubMed
-
- Whitehead V.M. (2006) Acquired and inherited disorders of cobalamin and folate in children. Br. J. Haematol., 134, 125–136. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

