Temporal Role for MyD88 in a Model of Brucella-Induced Arthritis and Musculoskeletal Inflammation
- PMID: 28069819
- PMCID: PMC5328475
- DOI: 10.1128/IAI.00961-16
Temporal Role for MyD88 in a Model of Brucella-Induced Arthritis and Musculoskeletal Inflammation
Abstract
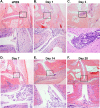
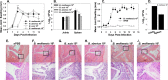
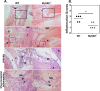
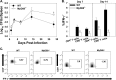
Brucella spp. are facultative intracellular Gram-negative bacteria that cause the zoonotic disease brucellosis, one of the most common global zoonoses. Osteomyelitis, arthritis, and musculoskeletal inflammation are common focal complications of brucellosis in humans; however, wild-type (WT) mice infected systemically with conventional doses of Brucella do not develop these complications. Here we report C57BL/6 WT mice infected via the footpad with 103 to 106 CFU of Brucella spp. display neutrophil and monocyte infiltration of the joint space and surrounding musculoskeletal tissue. Joint inflammation is detectable as early as 1 day postinfection and peaks 1 to 2 weeks later, after which WT mice are able to slowly resolve inflammation. B and T cells were dispensable for the onset of swelling but required for resolution of joint inflammation and infection. At early time points, MyD88-/- mice display decreased joint inflammation, swelling, and proinflammatory cytokine levels relative to WT mice. Subsequently, swelling of MyD88-/- joints surpassed WT joint swelling, and resolution of joint inflammation was prolonged. Joint bacterial loads in MyD88-/- mice were significantly greater than those in WT mice by day 3 postinfection and at all time points thereafter. In addition, MyD88-/- joint inflammatory cytokine levels on day 3 and beyond were similar to WT levels. Collectively these data demonstrate MyD88 signaling mediates early inflammatory responses in the joint but also contributes to subsequent clearance of Brucella and resolution of inflammation. This work also establishes a mouse model for studying Brucella-induced arthritis, musculoskeletal complications, and systemic responses, which will lead to a better understanding of focal complications of brucellosis.
Keywords: Brucella; MyD88; arthritis; brucellosis; musculoskeletal; osteomyelitis.
Copyright © 2017 American Society for Microbiology.
Figures



References
-
- . 2012. Research priorities for zoonoses and marginalized infections. World Health Organ Tech Rep Ser 2012:ix–xi, 1–119, 2 p following p 119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

