Determination of ∆-9-Tetrahydrocannabinol (THC), 11-hydroxy-THC, 11-nor-9-carboxy-THC and Cannabidiol in Human Plasma using Gas Chromatography-Tandem Mass Spectrometry
- PMID: 28069869
- PMCID: PMC5412026
- DOI: 10.1093/jat/bkw136
Determination of ∆-9-Tetrahydrocannabinol (THC), 11-hydroxy-THC, 11-nor-9-carboxy-THC and Cannabidiol in Human Plasma using Gas Chromatography-Tandem Mass Spectrometry
Abstract
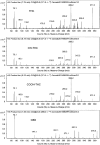
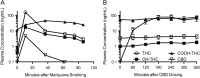
Two marijuana compounds of particular medical interest are delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). A gas chromatography-tandem mass spectrometry (GC-MS-MS) method was developed to test for CBD, THC, hydroxy-THC (OH-THC) and carboxy-THC (COOH-THC) in human plasma. Calibrators (THC and OH-THC, 0.1 to 100; CBD, 0.25 to 100; COOH-THC, 0.5-500 ng/mL) and controls (0.3, 5 and 80 ng/mL, except COOH-THC at 1.5, 25 and 400 ng/mL) were prepared in blank matrix. Deuterated (d3) internal standards were added to 1-mL samples. Preparation involved acetonitrile precipitation, liquid-liquid extraction (hexane:ethyl acetate, 9:1), and MSTFA derivatization. An Agilent 7890 A GC was interfaced with an Agilent 7000 MS Triple Quadrupole. Selected reaction monitoring was employed. Blood samples were provided from a marijuana smoking study (two participants) and a CBD ingestion study (eight participants). Three analytes with the same transitions (THC, OH-THC and COOH-THC) were chromatographically separated. Matrix selectivity studies showed endogenous chromatographic peak area ratios (PAR) at the analyte retention times were <20% of the analyte limit of quantitation PAR. The intra-assay accuracy ranged from 83.5% to 118% of target and the intra-run imprecision ranged from 2.0% to 19.1%. The inter-assay accuracy ranged from 90.3% to 104% of target and the inter-run imprecision ranged from 6.5% to 12.0%. Stability was established for 25 hours at room temperature, 207 days at -20°C, after three freeze-thaw cycles and for 26 days for rederivatized processed samples. After smoking marijuana predictable concentrations of THC, OH-THC and COOH-THC were seen; low concentrations of CBD were detected at early time points. In moderate users who had not smoked for at least 9 hours before ingesting an 800 mg oral dose of CBD, the method was sensitive enough to follow residual concentrations of THC and OH-THC; sustained COOH-THC concentrations over 50 ng/mL validated its higher analytical range.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Turner C.E., Elsohly M.A., Boeren E.G. (1980) Constituents of Cannabis sativa L. XVII. A review of the natural constituents. Journal of Natural Products, 43, 169–234. - PubMed
-
- Agurell S., Halldin M., Lindgren J.-E., Ohlson A., Widman M., Gillespie H., et al. (1986) Pharmacokineitcs and metabolism of ∆1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacological Reviews, 38, 21–43. - PubMed
-
- Jones R.T. (1987) Drug of abuse profile: cannabis. Clinical Chemistry, 33, 72B–81B. - PubMed
-
- Borgelt L.M., Franson K.L., Nussbaum A.M., Wang G.S. (2013) The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy, 33, 195–209. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

