Chronic innate immune activation of TBK1 suppresses mTORC1 activity and dysregulates cellular metabolism
- PMID: 28069950
- PMCID: PMC5278463
- DOI: 10.1073/pnas.1611113114
Chronic innate immune activation of TBK1 suppresses mTORC1 activity and dysregulates cellular metabolism
Abstract
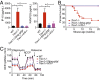
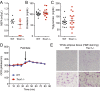
Three-prime repair exonuclease 1 knockout (Trex1-/-) mice suffer from systemic inflammation caused largely by chronic activation of the cyclic GMP-AMP synthase-stimulator of interferon genes-TANK-binding kinase-interferon regulatory factor 3 (cGAS-STING-TBK1-IRF3) signaling pathway. We showed previously that Trex1-deficient cells have reduced mammalian target of rapamycin complex 1 (mTORC1) activity, although the underlying mechanism is unclear. Here, we performed detailed metabolic analysis in Trex1-/- mice and cells that revealed both cellular and systemic metabolic defects, including reduced mitochondrial respiration and increased glycolysis, energy expenditure, and fat metabolism. We also genetically separated the inflammatory and metabolic phenotypes by showing that Sting deficiency rescued both inflammatory and metabolic phenotypes, whereas Irf3 deficiency only rescued inflammation on the Trex1-/- background, and many metabolic defects persist in Trex1-/-Irf3-/- cells and mice. We also showed that Leptin deficiency (ob/ob) increased lipogenesis and prolonged survival of Trex1-/- mice without dampening inflammation. Mechanistically, we identified TBK1 as a key regulator of mTORC1 activity in Trex1-/- cells. Together, our data demonstrate that chronic innate immune activation of TBK1 suppresses mTORC1 activity, leading to dysregulated cellular metabolism.
Keywords: TBK1; TREX1; innate immunity; mTORC1; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

