Injectable alginate-microencapsulated canine adipose tissue-derived mesenchymal stem cells for enhanced viable cell retention
- PMID: 28070061
- PMCID: PMC5383167
- DOI: 10.1292/jvms.16-0456
Injectable alginate-microencapsulated canine adipose tissue-derived mesenchymal stem cells for enhanced viable cell retention
Abstract
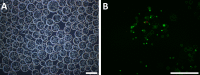
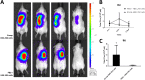
The purpose of this study was to establish an optimized protocol for the production of alginate-encapsulated canine adipose-derived mesenchymal stem cells (cASCs) and evaluate their suitability for clinical use, including viability, proliferation and in vivo cell retention. Alginate microbeads were formed by vibrational technology and the production of injectable microbeads was performed using various parameters with standard methodology. Microbead toxicity was tested in an animal model. Encapsulated cASCs were evaluated for viability and proliferation in vitro. HEK-293 cells, with or without microencapsulation, were injected into the subcutaneous tissue of mice and were tracked using in vivo bioluminescent imaging to evaluate the retention of transplanted cells. The optimized injectable microbeads were of uniform size and approximately 250 µm in diameter. There was no strong evidence of in vivo toxicity for the alginate beads. The cells remained viable after encapsulation, and there was evidence of in vitro proliferation within the microcapsules. In vivo bioluminescent imaging showed that alginate encapsulation improved the retention of transplanted cells and the encapsulated cells remained viable in vivo for 7 days. Encapsulation enhances the retention of viable cells in vivo and might represent a potential strategy to increase the therapeutic potency and efficacy of stem cells.
Figures




References
-
- Abbah S. A., Lu W. W., Chan D., Cheung K. M., Liu W. G., Zhao F., Li Z. Y., Leong J. C., Luk K. D.2006. In vitro evaluation of alginate encapsulated adipose-tissue stromal cells for use as injectable bone graft substitute. Biochem. Biophys. Res. Commun. 347: 185–191. doi: 10.1016/j.bbrc.2006.06.072 - DOI - PubMed
-
- Capone S. H., Dufresne M., Rechel M., Fleury M. J., Salsac A. V., Paullier P., Daujat-Chavanieu M., Legallais C.2013. Impact of alginate composition: from bead mechanical properties to encapsulated HepG2/C3A cell activities for in vivo implantation. PLoS ONE 8: e62032. doi: 10.1371/journal.pone.0062032 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

