Role of Uric Acid Metabolism-Related Inflammation in the Pathogenesis of Metabolic Syndrome Components Such as Atherosclerosis and Nonalcoholic Steatohepatitis
- PMID: 28070145
- PMCID: PMC5192336
- DOI: 10.1155/2016/8603164
Role of Uric Acid Metabolism-Related Inflammation in the Pathogenesis of Metabolic Syndrome Components Such as Atherosclerosis and Nonalcoholic Steatohepatitis
Abstract
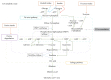
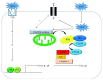
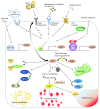
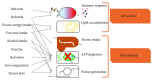
Uric acid (UA) is the end product of purine metabolism and can reportedly act as an antioxidant. However, recently, numerous clinical and basic research approaches have revealed close associations of hyperuricemia with several disorders, particularly those comprising the metabolic syndrome. In this review, we first outline the two molecular mechanisms underlying inflammation occurrence in relation to UA metabolism; one is inflammasome activation by UA crystallization and the other involves superoxide free radicals generated by xanthine oxidase (XO). Importantly, recent studies have demonstrated the therapeutic or preventive effects of XO inhibitors against atherosclerosis and nonalcoholic steatohepatitis, which were not previously considered to be related, at least not directly, to hyperuricemia. Such beneficial effects of XO inhibitors have been reported for other organs including the kidneys and the heart. Thus, a major portion of this review focuses on the relationships between UA metabolism and the development of atherosclerosis, nonalcoholic steatohepatitis, and related disorders. Although further studies are necessary, XO inhibitors are a potentially novel strategy for reducing the risk of many forms of organ failure characteristic of the metabolic syndrome.
Conflict of interest statement
The authors have no competing interests regarding the publication of this report to declare.
Figures
References
-
- Ames B. N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. - DOI - PMC - PubMed
-
- Kamogawa E., Sueishi Y. A multiple free-radical scavenging (MULTIS) study on the antioxidant capacity of a neuroprotective drug, edaravone as compared with uric acid, glutathione, and trolox. Bioorganic & Medicinal Chemistry Letters. 2014;24(5):1376–1379. doi: 10.1016/j.bmcl.2014.01.045. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

