Airway autoimmune responses in severe eosinophilic asthma following low-dose Mepolizumab therapy
- PMID: 28070196
- PMCID: PMC5216532
- DOI: 10.1186/s13223-016-0174-5
Airway autoimmune responses in severe eosinophilic asthma following low-dose Mepolizumab therapy
Abstract
Background: Anti-interleukin (IL)-5 monoclonal antibodies as an eosinophil-depleting strategy is well established, with Mepolizumab being the first biologic approved as an adjunct treatment for severe eosinophilic asthma.
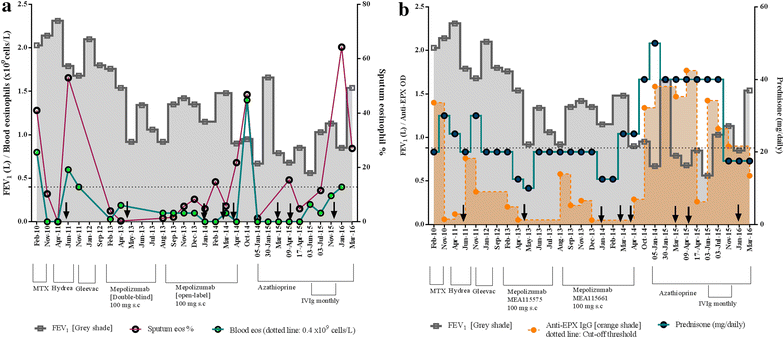
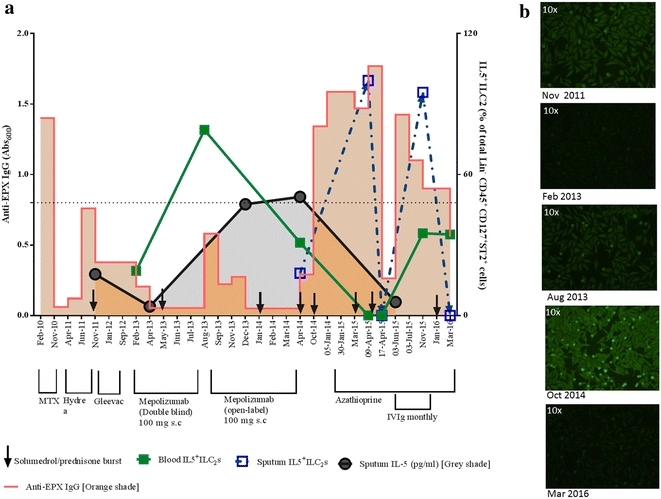
Case presentation: A 62-year old woman diagnosed with severe eosinophilic asthma showed poor response to Mepolizumab therapy (100 mg subcutaneous dose/monthly) and subsequent worsening of symptoms. The treatment response to Mepolizumab was monitored using both blood and sputum eosinophil counts. The latter was superior in assessing deterioration in symptoms, suggesting that normal blood eosinophil count may not always indicate amelioration or adequate control of the ongoing eosinophil-driven disease process. This perplexing situation of persistent airway eosinophilia and increased steroid insensitivity despite an anti-eosinophil therapy can be explained if the administered dose of the mAb was inadequate in comparison to the target antigen. The resultant immune complexes could act as 'cytokine depots', protecting the potency of the 'bound' IL-5, thereby sustaining the eosinophilic inflammation within the target tissue. Molecular analysis of the sputum indicated the development of a polyclonal autoimmune response as well as an increase in group 2 innate lymphoid cells, two novel observations in severe eosinophilic asthma, which were associated with indices of disease severity and progression. This case highlights the possibility of a previously unrecognised autoimmune-mediated worsening of asthma perhaps triggered by immune complexes formed due to inadequate dosing of administered monoclonal antibodies in the target tissue.
Conclusions: While anti-IL5 mAb therapy is an exciting novel option to treat patients with severe asthma, there is the rare possibility of worsening of asthma as observed in this case study, due to local autoimmune mechanisms precipitated by potential inadequate airway levels of the monoclonal antibody.
Keywords: Autoantibodies; Autoimmune; Eosinophilic asthma; IL-5; Immune complex; Mepolizumab; Sputum.
Figures
References
-
- FDA approves Nucala to treat severe asthma. US Food and Drug Administration: US Department of Health and Human Services; 2015. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm471031.htm. Accessed 10 Sept 2016.
-
- Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM, et al. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993;151:1235–1244. - PubMed
-
- Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O’Byrne PM, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137(1):75–86.e8. doi: 10.1016/j.jaci.2015.05.037. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

