Coordinated Plasticity between Barrel Cortical Glutamatergic and GABAergic Neurons during Associative Memory
- PMID: 28070425
- PMCID: PMC5192352
- DOI: 10.1155/2016/5648390
Coordinated Plasticity between Barrel Cortical Glutamatergic and GABAergic Neurons during Associative Memory
Abstract
Neural plasticity is associated with memory formation. The coordinated refinement and interaction between cortical glutamatergic and GABAergic neurons remain elusive in associative memory, which we examine in a mouse model of associative learning. In the mice that show odorant-induced whisker motion after pairing whisker and odor stimulations, the barrel cortical glutamatergic and GABAergic neurons are recruited to encode the newly learnt odor signal alongside the innate whisker signal. These glutamatergic neurons are functionally upregulated, and GABAergic neurons are refined in a homeostatic manner. The mutual innervations between these glutamatergic and GABAergic neurons are upregulated. The analyses by high throughput sequencing show that certain microRNAs related to regulating synapses and neurons are involved in this cross-modal reflex. Thus, the coactivation of the sensory cortices through epigenetic processes recruits their glutamatergic and GABAergic neurons to be the associative memory cells as well as drive their coordinated refinements toward the optimal state for the storage of the associated signals.
Conflict of interest statement
All authors declare that they have no competing interests.
Figures

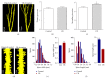

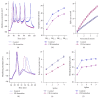


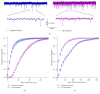
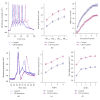

References
-
- Byrne J. H. Cellular analysis of associative learning. Physiological Reviews. 1987;67(2):329–439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

