Advanced MRI assessment to predict benefit of anti-programmed cell death 1 protein immunotherapy response in patients with recurrent glioblastoma
- PMID: 28070598
- PMCID: PMC6097616
- DOI: 10.1007/s00234-016-1769-8
Advanced MRI assessment to predict benefit of anti-programmed cell death 1 protein immunotherapy response in patients with recurrent glioblastoma
Abstract
Introduction: We describe the imaging findings encountered in GBM patients receiving immune checkpoint blockade and assess the potential of quantitative MRI biomarkers to differentiate patients who derive therapeutic benefit from those who do not.
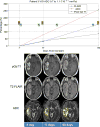
Methods: A retrospective analysis was performed on longitudinal MRIs obtained on recurrent GBM patients enrolled on clinical trials. Among 10 patients with analyzable data, bidirectional diameters were measured on contrast enhanced T1 (pGd-T1WI) and volumes of interest (VOI) representing measurable abnormality suggestive of tumor were selected on pGdT1WI (pGdT1 VOI), FLAIR-T2WI (FLAIR VOI), and ADC maps. Intermediate ADC (IADC) VOI represented voxels within the FLAIR VOI having ADC in the range of highly cellular tumor (0.7-1.1 × 10-3 mm2/s) (IADC VOI). Therapeutic benefit was determined by tissue pathology and survival on trial. IADC VOI, pGdT1 VOI, FLAIR VOI, and RANO assessment results were correlated with patient benefit.
Results: Five patients were deemed to have received therapeutic benefit and the other five patients did not. The average time on trial for the benefit group was 194 days, as compared to 81 days for the no benefit group. IADC VOI correlated well with the presence or absence of clinical benefit in 10 patients. Furthermore, pGd VOI, FLAIR VOI, and RANO assessment correlated less well with response.
Conclusion: MRI reveals an initial increase in volumes of abnormal tissue with contrast enhancement, edema, and intermediate ADC suggesting hypercellularity within the first 0-6 months of immunotherapy. Subsequent stabilization and improvement in IADC VOI appear to better predict ultimate therapeutic benefit from these agents than conventional imaging.
Keywords: ADC; GBM; Immunotherapy; MRI; Pseudoprogression.
Figures




References
-
- DeAngelis LM, Wen PY. Primary and metastatic tumors of the nervous system. In: Longo DL, Kasper DL, Hauser SL, Jameson J, Loscalzo J, editors. Harrison’s principles of internal medicine. 2012.
-
- Avril T, Vauleon E, Tanguy-Royer S, Mosser J, Quillien V. Mechanisms of immunomodulation in human glioblastoma. Immunotherapy. 2011;3:42–44. - PubMed
-
- Reardon DA, Gokhale PC, Klein SR, et al. Glioblastomaeradication following immune checkpoint blockade in an orthotopic, Immunocompetentmodel. Cancer Immunol Res. 2016;4:124–135. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

