"How to" incorporate dual-energy imaging into a high volume abdominal imaging practice
- PMID: 28070657
- PMCID: PMC5436906
- DOI: 10.1007/s00261-016-1035-x
"How to" incorporate dual-energy imaging into a high volume abdominal imaging practice
Abstract
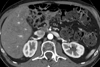
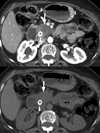
Dual-energy CT imaging has many potential uses in abdominal imaging. It also has unique requirements for protocol creation depending on the dual-energy scanning technique that is being utilized. It also generates several new types of images which can increase the complexity of image creation and image interpretation. The purpose of this article is to review, for rapid switching and dual-source dual-energy platforms, methods for creating dual-energy protocols, different approaches for efficiently creating dual-energy images, and an approach to navigating and using dual-energy images at the reading station all using the example of a pancreatic multiphasic protocol. It will also review the three most commonly used types of dual-energy images: "workhorse" 120kVp surrogate images (including blended polychromatic and 70 keV monochromatic), high contrast images (e.g., low energy monochromatic and iodine material decomposition images), and virtual unenhanced images. Recent developments, such as the ability to create automatically on the scanner the most common dual-energy images types, namely new "Mono+" images for the DSDECT (dual-source dual-energy CT) platform will also be addressed. Finally, an approach to image interpretation using automated "hanging protocols" will also be covered. Successful dual-energy implementation in a high volume practice requires careful attention to each of these steps of scanning, image creation, and image interpretation.
Keywords: Computed tomography; Dual-energy; Workflow.
Conflict of interest statement
Eric P. Tamm, Conflict of Interest: General Electric healthcare “in kind” research support.
Dianna D. Cody, Conflict of Interest: General Electric healthcare “in kind” research support. Philips Healthcare scientific advisory board. ACR CT accreditation reviewer.
Figures

















References
-
- Shuman WP, Green DE, Busey JM, et al. Dual-Energy Liver CT: Effect of Monochromatic Imaging on Lesion Detection, Conspicuity, and Contrast-to-Noise Ratio of Hypervascular Lesions on Late Arterial Phase. AJR Am J Roentgenol. 2014;203:601–606. - PubMed
-
- Patel BN, Thomas JV, Lockhart ME, et al. Single-source dual-energy spectral multidetector CT of pancreatic adenocarcinoma: optimization of energy level viewing significantly increases lesion contrast. Clin Radiol. 2013;68:148–154. - PubMed
-
- Lin XZ, Wu ZY, Tao R, et al. Dual energy spectral CT imaging of insulinoma-Value in preoperative diagnosis compared with conventional multi-detector CT. Eur J Radiol. 2012;81:2487–2494. - PubMed
-
- Apfaltrer P, Sudarski S, Schneider D, et al. Value of monoenergetic low-kV dual energy CT datasets for improved image quality of CT pulmonary angiography. Eur J Radiol. 2014;83:322–328. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

