Exosome and Microvesicle-Enriched Fractions Isolated from Mesenchymal Stem Cells by Gradient Separation Showed Different Molecular Signatures and Functions on Renal Tubular Epithelial Cells
- PMID: 28070858
- PMCID: PMC5380712
- DOI: 10.1007/s12015-016-9713-1
Exosome and Microvesicle-Enriched Fractions Isolated from Mesenchymal Stem Cells by Gradient Separation Showed Different Molecular Signatures and Functions on Renal Tubular Epithelial Cells
Abstract
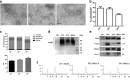
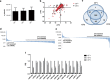
Several studies have suggested that extracellular vesicles (EVs) released from mesenchymal stem cells (MSCs) may mediate MSC paracrine action on kidney regeneration. This activity has been, at least in part, ascribed to the transfer of proteins/transcription factors and different RNA species. Information on the RNA/protein content of different MSC EV subpopulations and the correlation with their biological activity is currently incomplete. The aim of this study was to evaluate the molecular composition and the functional properties on renal target cells of MSC EV sub-populations separated by gradient floatation. The results demonstrated heterogeneity in quantity and composition of MSC EVs. Two peaks of diameter were observed (90-110 and 170-190 nm). The distribution of exosomal markers and miRNAs evaluated in the twelve gradient fractions showed an enrichment in fractions with a flotation density of 1.08-1.14 g/mL. Based on this observation, we evaluated the biological activity on renal cell proliferation and apoptosis resistance of low (CF1), medium (CF2) and high (CF3) floatation density fractions. EVs derived from all fractions, were internalized by renal cells, CF1 and CF2 but not CF3 fraction stimulated significant cell proliferation. CF2 also inhibited apoptosis on renal tubular cells submitted to ischemia-reperfusion injury. Comparative miRNomic and proteomic profiles reveal a cluster of miRNAs and proteins common to all three fractions and an enrichment of selected molecules related to renal regeneration in CF2 fraction. In conclusion, the CF2 fraction enriched in exosomal markers was the most active on renal tubular cell proliferation and protection from apoptosis.
Keywords: Acute kidney injury; Exosomes; Extracellular vesicles; Kidney regeneration; Mesenchymal stem cells; Microvesicles.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures

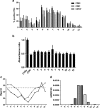
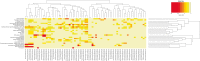
 ) shows its expression in almost all the EV fractions. On the contrary, miR-451 (
) shows its expression in almost all the EV fractions. On the contrary, miR-451 ( ) shows selective compartmentalization in the central fractions. d Representative qRT-PCR analysis showing the relative quantity of miR-451 in respect to the synthetic spike-in (UniSp6) used as normalizer. Normalized data showed the distribution of miR-451 inside the exosome-enriched EV fractions. Three experiments were conducted with similar results
) shows selective compartmentalization in the central fractions. d Representative qRT-PCR analysis showing the relative quantity of miR-451 in respect to the synthetic spike-in (UniSp6) used as normalizer. Normalized data showed the distribution of miR-451 inside the exosome-enriched EV fractions. Three experiments were conducted with similar results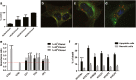
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

