Convergent gustatory and viscerosensory processing in the human dorsal mid-insula
- PMID: 28070928
- PMCID: PMC5575915
- DOI: 10.1002/hbm.23510
Convergent gustatory and viscerosensory processing in the human dorsal mid-insula
Abstract
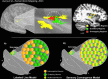
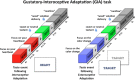
The homeostatic regulation of feeding behavior requires an organism to be able to integrate information from its internal environment, including peripheral visceral signals about the body's current energy needs, with information from its external environment, such as the palatability of energy-rich food stimuli. The insula, which serves as the brain's primary sensory cortex for representing both visceral signals from the body and taste signals from the mouth and tongue, is a likely candidate region in which this integration might occur. However, to date it has been unclear whether information from these two homeostatically critical faculties is merely co-represented in the human insula, or actually integrated there. Recent functional neuroimaging evidence of a common substrate for visceral interoception and taste perception within the human dorsal mid-insula suggests a model whereby a single population of neurons may integrate viscerosensory and gustatory signals. To test this model, we used fMRI-Adaptation to identify whether insula regions that exhibit repetition suppression following repeated interoception trials would then also exhibit adapted responses to subsequent gustatory stimuli. Multiple mid and anterior regions of the insula exhibited adaptation to interoceptive trials specifically, but only the dorsal mid-insula regions exhibited an adapted gustatory response following interoception. The discovery of this gustatory-interoceptive convergence within the neurons of the human insula supports the existence of a heretofore-undocumented neural pathway by which visceral signals from the periphery modulate the activity of brain regions involved in feeding behavior. Hum Brain Mapp 38:2150-2164, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: fMRI; fMRI-adaptation; gustation; insular cortex; interoception.
© 2017 Wiley Periodicals, Inc.
Figures



References
-
- Berthoud HR, Neuhuber WL (2000): Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85:1–17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

