Neuronal sFlt1 and Vegfaa determine venous sprouting and spinal cord vascularization
- PMID: 28071661
- PMCID: PMC5234075
- DOI: 10.1038/ncomms13991
Neuronal sFlt1 and Vegfaa determine venous sprouting and spinal cord vascularization
Abstract
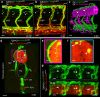
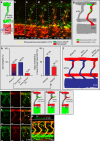
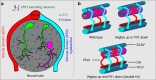
Formation of organ-specific vasculatures requires cross-talk between developing tissue and specialized endothelial cells. Here we show how developing zebrafish spinal cord neurons coordinate vessel growth through balancing of neuron-derived Vegfaa, with neuronal sFlt1 restricting Vegfaa-Kdrl mediated angiogenesis at the neurovascular interface. Neuron-specific loss of flt1 or increased neuronal vegfaa expression promotes angiogenesis and peri-neural tube vascular network formation. Combining loss of neuronal flt1 with gain of vegfaa promotes sprout invasion into the neural tube. On loss of neuronal flt1, ectopic sprouts emanate from veins involving special angiogenic cell behaviours including nuclear positioning and a molecular signature distinct from primary arterial or secondary venous sprouting. Manipulation of arteriovenous identity or Notch signalling established that ectopic sprouting in flt1 mutants requires venous endothelium. Conceptually, our data suggest that spinal cord vascularization proceeds from veins involving two-tiered regulation of neuronal sFlt1 and Vegfaa via a novel sprouting mode.
Figures






References
-
- Zacchigna S., Lambrechts D. & Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 9, 169–181 (2008). - PubMed
-
- Eichmann A., Le Noble F., Autiero M. & Carmeliet P. Guidance of vascular and neural network formation. Curr. Opin. Neurobiol. 15, 108–115 (2005). - PubMed
-
- Larrivee B., Freitas C., Suchting S., Brunet I. & Eichmann A. Guidance of vascular development: Lessons from the nervous system. Circ. Res. 104, 428–441 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

