Purification and differentiation of human adipose-derived stem cells by membrane filtration and membrane migration methods
- PMID: 28071738
- PMCID: PMC5223180
- DOI: 10.1038/srep40069
Purification and differentiation of human adipose-derived stem cells by membrane filtration and membrane migration methods
Abstract
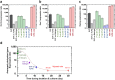
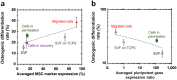
Human adipose-derived stem cells (hADSCs) are easily isolated from fat tissue without ethical concerns, but differ in purity, pluripotency, differentiation ability, and stem cell marker expression, depending on the isolation method. We isolated hADSCs from a primary fat tissue solution using: (1) conventional culture, (2) a membrane filtration method, (3) a membrane migration method where the primary cell solution was permeated through membranes, adhered hADSCs were cultured, and hADSCs migrated out from the membranes. Expression of mesenchymal stem cell markers and pluripotency genes, and osteogenic differentiation were compared for hADSCs isolated by different methods using nylon mesh filter membranes with pore sizes ranging from 11 to 80 μm. hADSCs isolated by the membrane migration method had the highest MSC surface marker expression and efficient differentiation into osteoblasts. Osteogenic differentiation ability of hADSCs and MSC surface marker expression were correlated, but osteogenic differentiation ability and pluripotent gene expression were not.
Figures





References
-
- Takahashi K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–72 (2007). - PubMed
-
- Higuchi A. et al. Generation of pluripotent stem cells without the use of genetic material. Lab. Invest. 95, 26–42 (2015). - PubMed
-
- Yu J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007). - PubMed
-
- Higuchi A. et al. Biomaterials for the feeder-free culture of human embryonic stem cells and induced pluripotent stem cells. Chemical Reviews 111, 3021–35 (2011). - PubMed
-
- Thomson J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

