Evidence that interfibrillar load transfer in tendon is supported by small diameter fibrils and not extrafibrillar tissue components
- PMID: 28071819
- PMCID: PMC5503823
- DOI: 10.1002/jor.23517
Evidence that interfibrillar load transfer in tendon is supported by small diameter fibrils and not extrafibrillar tissue components
Abstract
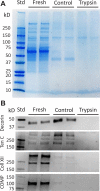
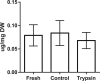
Collagen fibrils in tendon are believed to be discontinuous and transfer tensile loads through shear forces generated during interfibrillar sliding. However, the structures that transmit these interfibrillar forces are unknown. Various extrafibrillar tissue components (e.g., glycosaminoglycans, collagens XII and XIV) have been suggested to transmit interfibrillar loads by bridging collagen fibrils. Alternatively, collagen fibrils may interact directly through physical fusions and interfibrillar branching. The objective of this study was to test whether extrafibrillar proteins are necessary to transmit load between collagen fibrils or if interfibrillar load transfer is accomplished directly by the fibrils themselves. Trypsin digestions were used to remove a broad spectrum of extrafibrillar proteins and measure their contribution to the multiscale mechanics of rat tail tendon fascicles. Additionally, images obtained from serial block-face scanning electron microscopy were used to determine the three-dimensional fibrillar organization in tendon fascicles and identify any potential interfibrillar interactions. While trypsin successfully removed several extrafibrillar tissue components, there was no change in the macroscale fascicle mechanics or fibril:tissue strain ratio. Furthermore, the imaging data suggested that a network of smaller diameter fibrils (<150 nm) wind around and fuse with their neighboring larger diameter fibrils. These findings demonstrate that interfibrillar load transfer is not supported by extrafibrillar tissue components and support the hypothesis that collagen fibrils are capable of transmitting loads themselves. Conclusively determining how fibrils bear load within tendon is critical for identifying the mechanisms that impair tissue function with degeneration and for restoring tissue properties via cell-mediated regeneration or engineered tissue replacements. © 2017 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 35:2127-2134, 2017.
Keywords: extrafibrillar proteins; interfibrillar load transfer; multiscale mechanics; tendon; trypsin digestion.
© 2017 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures

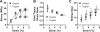


References
-
- Screen HRC, Lee DA, Bader DL, Shelton JC. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. [2012 May 9];Proc. Inst. Mech. Eng. [H] 2004 218(2):109–119. - PubMed
-
- Rigozzi S, Stemmer A, Müller R, Snedeker JG. Mechanical response of individual collagen fibrils in loaded tendon as measured by atomic force microscopy. [2012 May 9];J. Struct. Biol. 2011 176(1):9–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

