The making of bispecific antibodies
- PMID: 28071970
- PMCID: PMC5297537
- DOI: 10.1080/19420862.2016.1268307
The making of bispecific antibodies
Abstract
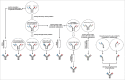
During the past two decades we have seen a phenomenal evolution of bispecific antibodies for therapeutic applications. The 'zoo' of bispecific antibodies is populated by many different species, comprising around 100 different formats, including small molecules composed solely of the antigen-binding sites of two antibodies, molecules with an IgG structure, and large complex molecules composed of different antigen-binding moieties often combined with dimerization modules. The application of sophisticated molecular design and genetic engineering has solved many of the technical problems associated with the formation of bispecific antibodies such as stability, solubility and other parameters that confer drug properties. These parameters may be summarized under the term 'developability'. In addition, different 'target product profiles', i.e., desired features of the bispecific antibody to be generated, mandates the need for access to a diverse panel of formats. These may vary in size, arrangement, valencies, flexibility and geometry of their binding modules, as well as in their distribution and pharmacokinetic properties. There is not 'one best format' for generating bispecific antibodies, and no single format is suitable for all, or even most of, the desired applications. Instead, the bispecific formats collectively serve as a valuable source of diversity that can be applied to the development of therapeutics for various indications. Here, a comprehensive overview of the different bispecific antibody formats is provided.
Keywords: Appended IgG; Fab; Fc heterodimerization; bispecific antibodies; fusion proteins; immunoglobulin; scFv.
Figures



References
-
- Dhimolea E, Reichert JM. World Bispecific Antibody Summit, September 27-28, 2011, Boston, MA. MAbs 2012; 4:4-13; PMID:22327426; http://dx.doi.org/ 10.4161/mabs.4.1.18821 - DOI - PMC - PubMed
-
- Garber K. Bispecific antibodies rise again. Nat Rev Drug Discov 2014; 13:799-801; PMID:25359367; http://dx.doi.org/ 10.1038/nrd4478 - DOI - PubMed
-
- Sheridan C. Amgen's bispecific antibody puffs across finish line. Nat Biotechnol 2015; 33:219-21; PMID:25748895; http://dx.doi.org/ 10.1038/nbt0315-219 - DOI - PubMed
-
- Nisonoff A, Rivers MM. Recombination of a mixture of univalent antibody fragments of different specificity. Arch Biochem Biophys 1961; 93:460-2; PMID:13729244 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
