BRAFV600E cooperates with CDX2 inactivation to promote serrated colorectal tumorigenesis
- PMID: 28072391
- PMCID: PMC5268782
- DOI: 10.7554/eLife.20331
BRAFV600E cooperates with CDX2 inactivation to promote serrated colorectal tumorigenesis
Abstract
While 20-30% of colorectal cancers (CRCs) may arise from precursors with serrated glands, only 8-10% of CRCs manifest serrated morphology at diagnosis. Markers for distinguishing CRCs arising from 'serrated' versus 'conventional adenoma' precursors are lacking. We studied 36 human serrated CRCs and found CDX2 loss or BRAF mutations in ~60% of cases and often together (p=0.04). CDX2Null/BRAFV600E expression in adult mouse intestinal epithelium led to serrated morphology tumors (including carcinomas) and BRAFV600E potently interacted with CDX2 silencing to alter gene expression. Like human serrated lesions, CDX2Null/BRAFV600E-mutant epithelium expressed gastric markers. Organoids from CDX2Null/BRAFV600E-mutant colon epithelium showed serrated features, and partially recapitulated the gene expression pattern in mouse colon tissues. We present a novel mouse tumor model based on signature defects seen in many human serrated CRCs - CDX2 loss and BRAFV600E. The mouse intestinal tumors show significant phenotypic similarities to human serrated CRCs and inform about serrated CRC pathogenesis.
Keywords: cancer biology; cancer diagnosis; colorectal cancer; homeobox gene; human; human biology; intestinal differentiation; medicine; mouse; oncogene; tumor suppressor gene.
Conflict of interest statement
MM: Reviewing editor, eLife. The other authors declare that no competing interests exist.
Figures

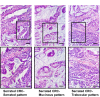


 indicates the ileum. Scale bar, 10 mm (C) Histological features of the proximal colon-cecum-ileum junction tumor of CDX2P-CreERT2Cdx2fl/fl BrafCA mice. Two major components were found in the tumor: serrated region (upper right) and mucin-rich region (lower left). Scale bars, 200 μm for low magnification image (left); 50 μm for high magnification images (right). (D) Serrated region showing invasion into submucosa. Dashed-line highlights muscular mucosa. Scale bar, 100 μm. See also Figure 2—source data 1 for panel A. DOI:
indicates the ileum. Scale bar, 10 mm (C) Histological features of the proximal colon-cecum-ileum junction tumor of CDX2P-CreERT2Cdx2fl/fl BrafCA mice. Two major components were found in the tumor: serrated region (upper right) and mucin-rich region (lower left). Scale bars, 200 μm for low magnification image (left); 50 μm for high magnification images (right). (D) Serrated region showing invasion into submucosa. Dashed-line highlights muscular mucosa. Scale bar, 100 μm. See also Figure 2—source data 1 for panel A. DOI:










References
-
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

