Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients
- PMID: 28073178
- PMCID: PMC6464766
- DOI: 10.1002/14651858.CD004759.pub2
Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients
Abstract
Background: Prolonging kidney transplant survival is an important clinical priority. Induction immunosuppression with antibody therapy is recommended at transplantation and non-depleting interleukin-2 receptor monoclonal antibodies (IL2Ra) are considered first line. It is suggested that recipients at high risk of rejection should receive lymphocyte-depleting antibodies but the relative benefits and harms of the available agents are uncertain.
Objectives: We aimed to: evaluate the relative and absolute effects of different antibody preparations (except IL2Ra) when used as induction therapy in kidney transplant recipients; determine how the benefits and adverse events vary for each antibody preparation; determine how the benefits and harms vary for different formulations of antibody preparation; and determine whether the benefits and harms vary in specific subgroups of recipients (e.g. children and sensitised recipients).
Search methods: Randomised controlled trials (RCTs) comparing monoclonal or polyclonal antibodies with placebo, no treatment, or other antibody therapy in adults and children who had received a kidney transplant.
Selection criteria: Randomised controlled trials (RCTs) comparing monoclonal or polyclonal antibodies with placebo, no treatment, or other antibody therapy in adults and children who had received a kidney transplant.
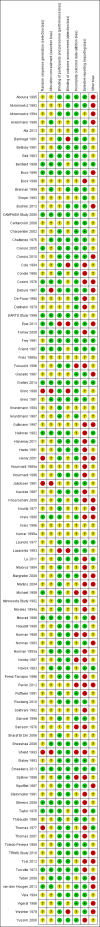
Data collection and analysis: Two authors independently extracted data and assessed risk of bias. Dichotomous outcomes are reported as relative risk (RR) and continuous outcomes as mean difference (MD) together with their 95% confidence intervals (CI).
Main results: We included 99 studies (269 records; 8956 participants; 33 with contemporary agents). Methodology was incompletely reported in most studies leading to lower confidence in the treatment estimates.Antithymocyte globulin (ATG) prevented acute graft rejection (17 studies: RR 0.63, 95% CI 0.51 to 0.78). The benefits of ATG on graft rejection were similar when used with (12 studies: RR 0.61, 0.49 to 0.76) or without (5 studies: RR 0.65, 0.43 to 0.98) calcineurin inhibitor (CNI) treatment. ATG (with CNI therapy) had uncertain effects on death (3 to 6 months, 3 studies: RR 0.41, 0.13 to 1.22; 1 to 2 years, 5 studies: RR 0.75, 0.27 to 2.06; 5 years, 2 studies: RR 0.94, 0.11 to 7.81) and graft loss (3 to 6 months, 4 studies: RR 0.60, 0.34 to 1.05; 1 to 2 years, 3 studies: RR 0.65, 0.36 to 1.19). The effect of ATG on death-censored graft loss was uncertain at 1 to 2 years and 5 years. In non-CNI studies, ATG had uncertain effects on death but reduced death-censored graft loss (6 studies: RR 0.55, 0.38 to 0.78). When CNI and older non-CNI studies were combined, a benefit was seen with ATG at 1 to 2 years for both all-cause graft loss (7 studies: RR 0.71, 0.53 to 0.95) and death-censored graft loss (8 studies: RR 0.55, 0.39 to 0.77) but not sustained longer term. ATG increased cytomegalovirus (CMV) infection (6 studies: RR 1.55, 1.24 to 1.95), leucopenia (4 studies: RR 3.86, 2.79 to 5.34) and thrombocytopenia (4 studies: RR 2.41, 1.61 to 3.61) but had uncertain effects on delayed graft function, malignancy, post-transplant lymphoproliferative disorder (PTLD), and new onset diabetes after transplantation (NODAT).Alemtuzumab was compared to ATG in six studies (446 patients) with early steroid withdrawal (ESW) or steroid minimisation. Alemtuzumab plus steroid minimisation reduced acute rejection compared to ATG at one year (4 studies: RR 0.57, 0.35 to 0.93). In the two studies with ESW only in the alemtuzumab arm, the effect of alemtuzumab on acute rejection at 1 year was uncertain compared to ATG (RR 1.27, 0.50 to 3.19). Alemtuzumab had uncertain effects on death (1 year, 2 studies: RR 0.39, 0.06 to 2.42; 2 to 3 years, 3 studies: RR 0.67, 95% CI 0.15 to 2.95), graft loss (1 year, 2 studies: RR 0.39, 0.13 to 1.30; 2 to 3 years, 3 studies: RR 0.98, 95% CI 0.47 to 2.06), and death-censored graft loss (1 year, 2 studies: RR 0.38, 0.08 to 1.81; 2 to 3 years, 3 studies: RR 2.45, 95% CI 0.67 to 8.97) compared to ATG. Creatinine clearance was lower with alemtuzumab plus ESW at 6 months (2 studies: MD -13.35 mL/min, -23.91 to -2.80) and 2 years (2 studies: MD -12.86 mL/min, -23.73 to -2.00) compared to ATG plus triple maintenance. Across all 6 studies, the effect of alemtuzumab versus ATG was uncertain on all-cause infection, CMV infection, BK virus infection, malignancy, and PTLD. The effect of alemtuzumab with steroid minimisation on NODAT was uncertain, compared to ATG with steroid maintenance.Alemtuzumab plus ESW compared with triple maintenance without induction therapy had uncertain effects on death and all-cause graft loss at 1 year, acute rejection at 6 months and 1 year. CMV infection was increased (2 studies: RR 2.28, 1.18 to 4.40). Treatment effects were uncertain for NODAT, thrombocytopenia, and malignancy or PTLD.Rituximab had uncertain effects on death, graft loss, acute rejection and all other adverse outcomes compared to placebo.
Authors' conclusions: ATG reduces acute rejection but has uncertain effects on death, graft survival, malignancy and NODAT, and increases CMV infection, thrombocytopenia and leucopenia. Given a 45% acute rejection risk without ATG induction, seven patients would need treatment to prevent one having rejection, while incurring an additional patient experiencing CMV disease for every 12 treated. Excluding non-CNI studies, the risk of rejection was 37% without induction with six patients needing treatment to prevent one having rejection.In the context of steroid minimisation, alemtuzumab prevents acute rejection at 1 year compared to ATG. Eleven patients would require treatment with alemtuzumab to prevent 1 having rejection, assuming a 21% rejection risk with ATG.Triple maintenance without induction therapy compared to alemtuzumab combined with ESW had similar rates of acute rejection but adverse effects including NODAT were poorly documented. Alemtuzumab plus steroid withdrawal would cause one additional patient experiencing CMV disease for every six patients treated compared to no induction and triple maintenance, in the absence of any clinical benefit. Overall, ATG and alemtuzumab decrease acute rejection at a cost of increased CMV disease while patient-centred outcomes (reduced death or lower toxicity) do not appear to be improved.
Conflict of interest statement
AW: Nothing to declare
NC: Nothing to declare
PH: Nothing to declare
NB: NB is a co‐investigator of the ongoing randomised, controlled clinical trial, ReMIND (RituxiMab INDuction in renal transplantation, NCT01095172).
SP: Nothing to declare
Figures
Update of
References
References to studies included in this review
Abouna 1995 {published data only}
-
- Abouna GM, Kumar MS, al‐Abdullah IH, Loose J, Sullivan DK, Phillips K, et al. Induction immunosuppression with antithymocyte globulin in renal transplantation using a variable dose according to the absolute number of CD3+ T cells. Transplantation Proceedings 1995;27(5):2676‐8. [MEDLINE: ] - PubMed
-
- Abouna GM, al‐Abdullah IH, Kelly‐Sullivan D, Kumar MS, Loose J, Phillips K, et al. Randomized clinical trial of antithymocyte globulin induction in renal transplantation comparing a fixed daily dose with dose adjustment according to T cell monitoring. Transplantation 1995;59(11):1564‐8. [MEDLINE: ] - PubMed
Abramowicz 1992 {published data only}
-
- Abramowicz D, Goldman M, Pauw L, Vanherweghem JL, Kinnaert P, Vereerstraeten P. The long‐term effects of prophylactic OKT3 monoclonal antibody in cadaver kidney transplantation‐‐a single‐center, prospective, randomized study. Transplantation 1992;54(3):433‐7. [MEDLINE: ] - PubMed
-
- Abramowicz D, Norman DJ, Goldman M, Pauw L, Kinnaert P, Kahana L, et al. OKT3 prophylaxis improves long‐term renal graft survival in high‐risk patients as compared to cyclosporine: combined results from the prospective, randomized Belgian and US studies. Transplantation Proceedings 1995;27(1):852‐3. [MEDLINE: ] - PubMed
-
- Abramowicz D, Norman DJ, Vereerstraeten P, Goldman M, Pauw L, Vanherweghem JL, et al. OKT3 prophylaxis in renal grafts with prolonged cold ischemia times: association with improvement in long‐term survival. Kidney International 1996;49(3):768‐72. [MEDLINE: ] - PubMed
-
- Goldman M, Abramowicz D, Pauw L, Marecaux G, Vanderwinden JM, Kinnaert P, et al. Beneficial effects of prophylactic OKT3 in cadaver kidney transplantation: comparison with cyclosporin A in a single‐center prospective randomized study. Transplantation Proceedings 1991;23(1 Pt 2):1046‐7. [MEDLINE: ] - PubMed
Abramowicz 1994 {published data only}
-
- Abramowicz D, Goldman M, Mat O, Estermans G, Crusiaux A, Vanherweghem JL, et al. OKT3 serum levels as a guide for prophylactic therapy: a pilot study in kidney transplant recipients. Transplant International 1994;7(4):258‐63. [MEDLINE: ] - PubMed
Ackermann 1988 {published data only}
-
- Ackermann JR, Lefor WM, Kahana L, Weinstein S, Shires DL. Prophylactic use of OKT3 in renal transplantation: Part of a prospective randomized multicenter trial. Transplantation Proceedings 1988;20(1 Suppl 1):242‐4. [EMBASE: 1988111881]
-
- Kahana L, Ackermann J, Lefor W, Weinstein S, Wright C, DeQuesada A, et al. Uses of orthoclone OKT3 for prophylaxis of rejection and induction in initial nonfunction in kidney transplantation. Transplantation Proceedings 1990;22(4):1755‐8. [MEDLINE: ] - PubMed
-
- Kahana L, Narvarte J, Ackermann J, Lefor W, Weinstein S, Wright C, et al. OKT3 prophylaxis versus conventional drug therapy: single‐center perspective, part of a multicenter trial. American Journal of Kidney Diseases 1989;14(5 Suppl 2):5‐9. [MEDLINE: ] - PubMed
Ata 2013 {published data only}
-
- Ata P, Kara M, Ozdemir E, Canbakan M, Gokce AM, Bayraktar FA, et al. Monitoring of CD3(+) T‐cell count in patients receiving antithymocyte globulin induction after cadaveric renal transplantation. Transplantation Proceedings 2013;45(3):929‐31. [MEDLINE: ] - PubMed
Banhegyi 1991 {published data only}
-
- Banhegyi C, Rockenschaub S, Muhlbacher F, Kovarik J, Balcke P, Gotzinger P, et al. Preliminary results of a prospective randomized clinical trial comparing cyclosporine A to antithymocyte globulin immunosuppressive induction therapy in kidney transplantation. Transplantation Proceedings 1991;23(4):2207‐8. [MEDLINE: ] - PubMed
Belitsky 1991 {published data only}
-
- Belitsky P, MacDonald AS, Cohen AD, Crocker J, Hirsch D, Jindal K, et al. Comparison of antilymphocyte globulin and continuous i.v. cyclosporine A as induction immunosuppression for cadaver kidney transplants: a prospective randomized study. Transplantation Proceedings 1991;23(1 Pt 2):999‐1000. [MEDLINE: ] - PubMed
-
- Gulanikar AC, MacDonald AS, Sungurtekin U, Belitsky P. The incidence and impact of early rejection episodes on graft outcome in recipients of first cadaver kidney transplants. Transplantation 1992;53(2):323‐8. [MEDLINE: ] - PubMed
-
- Gulanikar AC, Sungurtekin U, MacDonald AS, Belitsky P, Bitter‐Suermann H, Cohen A, et al. Sequential discontinuation of azathioprine and prednisone in renal transplantation. Transplantation Proceedings 1991;23(4):2226‐7. [MEDLINE: ] - PubMed
Bell 1983 {published data only}
-
- Bell PR, Blamey RW, Briggs JD, Castro JE, Hamilton DN, Knapp MS, et al. Medical research council trial of antilymphocyte globulin in renal transplantation. A multicenter randomized double‐blind placebo controlled clinical investigation. Transplantation 1983;35(6):539‐45. [MEDLINE: ] - PubMed
Benfield 1999 {published data only}
-
- Benfield MR, Herrin J, Feld L, Rose S, Stablein D, Tejani A. Safety of kidney biopsy in pediatric transplantation: a report of the Controlled Clinical Trials in Pediatric Transplantation Trial of Induction Therapy Study Group. Transplantation 1999;67(4):544‐7. [MEDLINE: ] - PubMed
-
- Benfield MR, Symons JM, Bynon S, Eckhoff D, Herrin J, Harmon W, et al. Mycophenolate mofetil in pediatric renal transplantation. Pediatric Transplantation 1999;3(1):33‐7. [MEDLINE: ] - PubMed
-
- Benfield MR, Tejani A, Harmon WE, McDonald R, Stablein DM, McIntosh M, et al. A randomized multicenter trial of OKT3 mAbs induction compared with intravenous cyclosporine in pediatric renal transplantation. Pediatric Transplantation 2005;9(3):282‐92. [MEDLINE: ] - PubMed
-
- Tejani A. A randomized prospective multicenter trial of T‐cell antibody induction therapy in pediatric renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000.
-
- Tejani A, Harmon W, Benfield M, Elshihabi I, McDonald R, Stablein D, et al. A randomized prospective multicenter trial of t‐cell antibody induction therapy in pediatric renal transplantation [abstract]. Transplantation 2000;69(8 Suppl):S111. [CENTRAL: CN‐00402826]
Bock 1995 {published data only}
-
- Bock HA, Gallati H, Zurcher RM, Bachofen M, Mihatsch MJ, Landmann J, et al. A randomized prospective trial of prophylactic immunosuppression with ATG‐Fresenius versus OKT3 after renal transplantation. Transplantation 1995;59(6):830‐40. [MEDLINE: ] - PubMed
-
- Bock HA, Zurcher R, Mihatsch M, Landmann J, Thiel G. A randomized prospective trial of prophylactic immunosuppression with OKT3 vs. ATG‐Fresenius (ATG‐F) after renal transplantation [abstract]. 12th International Congress of Nephrology; 1993 June 13‐18; Jerusalem, Israel. 1993:165. [CENTRAL: CN‐00550381]
-
- Bock HA, Zurcher R, Mihatsch M, Landmann J, Thiel G. A randomized prospective trial of prophylactic therapy with OKT3 vs ATG‐Fresenius (ATG‐F) after renal transplantation [abstract]. Journal of the American Society of Nephrology 1992;3(3):852. [CENTRAL: CN‐00460418]
Bock 1999 {published data only}
-
- Bock HA, Tsinalis D, Nickeleit V, Mihatsch M, Thiel G. Randomized prospective study of polyclonal antilymphocyte globulin induction after renal transplantation: ATGAM vs ATG‐Fresenius [abstract no: A3646]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):720A. [CENTRAL: CN‐00583882]
Brennan 1999 {published data only}
-
- Brennan DC, Flavin K, Burgess S, Dolan S, Kano JM, Mahon M, et al. A randomized, double‐blinded comparison of thymoglobulin vs ATGAM for induction in adult renal transplant recipients [abstract no: 710]. Transplantation 1998;65(12):S180. [CENTRAL: CN‐00602088] - PubMed
-
- Brennan DC, Flavin K, Lowell JA, Howard TK, Shenoy S, Burgess S, et al. A randomized, double‐blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. [erratum appears in Transplantation 1999 May 27;67(10):1386.]. Transplantation 1999;67(7):1011‐8. [MEDLINE: ] - PubMed
-
- Brennan DC, Flavin K, Lowell JA, Howard TK, Shenoy S, Burgess S, et al. Leukocyte response to thymoglobulin or ATGAM for induction immunosuppression in a randomized, double‐blind clinical trial in renal transplant recipients. Transplantation Proceedings 1999;31(3B Suppl):16S‐8S. [MEDLINE: ] - PubMed
-
- Hardinger KL, Schnitzler MA, Miller B, Lowell J, Shenoy S, Brennan DC. Long‐term comparison of thymoglobulin versus ATGAM for induction in adult renal transplantation: evidence of improved allograft survival at 5 years [abstract]. American Journal of Transplantation 2003;3(Suppl 5):556. [CENTRAL: CN‐00445642]
Broyer 1993 {published data only}
-
- Broyer M, Gagnadoux MF, Guest G, Arsan A, Beurton D, Revillon Y, et al. Prophylactic OKT3 monoclonal antibody versus antilymphocyte globulins: a prospective, randomized study in 148 first cadaver kidney grafts. Transplantation Proceedings 1993;25(1 Pt 1):570‐1. [MEDLINE: ] - PubMed
Buchler 2013 {published data only}
-
- Buchler M, Longuet H, Lemoine R, Herr F, Gatault P, Thibault G, et al. Pharmacokinetic and pharmacodynamic studies of two different rabbit antithymocyte globulin dosing regimens: results of a randomized trial. Transplant Immunology 2013;28(2‐3):120‐6. [MEDLINE: ] - PubMed
-
- Longuet H, Buchler M, Lemoine R, Herr F, Gatault P, Thibault G, et al. Pharmacokinetic and pharmacodynamic studies of two different rabbit antithymocyte antiglobulin dosing regimens [abstract no: O069]. Transplant International 2013;26(Suppl 2):44‐5. [EMBASE: 71359107] - PubMed
CAMPASIA Study 2005 {published data only}
-
- Munoz AS, Cabanayan‐Casasola CB, Danguilan RA, Padua FB, Ona ET. Campath‐1H (alemtuzumab) as an induction agent for the prevention of graft rejection and preservation of renal function in kidney transplant patients: Philippine 3‐year follow‐up. Transplantation Proceedings 2008;40(7):2230‐3. [MEDLINE: ] - PubMed
-
- Vathsala A, CAMPASIA Study Group. Safety and efficacy of campath‐1h (mabcampath®) with low dose cyclosprorine monotherapy in patients receiving kidney transplants ‐ 6 month analysis of the pilot randomised controlled [abstract]. Transplantation 2004;78(2 Suppl):56. [CENTRAL: CN‐00509538]
-
- Vathsala A, Campasia Study Group. One year results of a pilot randomised controlled trial of the effectiveness of alemtuzumab as an induction agent for prevention of graft rejection and preservation of renal function in patients receiving kidney transplants [abstract no: T‐PO50029]. Nephrology 2005;10(Suppl):A215. [CENTRAL: CN‐00583367]
-
- Vathsala A, Ona ET, Tan S, Suresh S, Chan Y, Lou H, et al. Campasia: a pilot randomised controlled trial of the effectiveness of campath‐1h (mabcampath®) as an induction agent for prevention of graft rejection and preservation of renal function in patients receiving kidney transplants [abstract]. American Journal of Transplantation 2004;4(Suppl 8):406. [CENTRAL: CN‐00509539]
-
- Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Cabanayan Casasola CB, et al. Randomized trial of Alemtuzumab for prevention of graft rejection and preservation of renal function after kidney transplantation. Transplantation 2005;80(6):765‐74. [MEDLINE: ] - PubMed
Cantarovich 2008 {published data only}
-
- Cantarovich M, Durrbach A, Hiesse C, Benoit G, Charpentier B. Short and long‐term impact of anti‐thymocyte globulin induction on clinical outcomes in renal transplant patients: 15‐year results of a prospective and randomized trial [abstract no: 1034]. American Journal of Transplantation 2002;2(Suppl 3):398. [CENTRAL: CN‐00415382]
-
- Cantarovich M, Durrbach A, Hiesse C, Ladouceur M, Benoit G, Charpentier B. 20‐year follow‐up results of a randomized controlled trial comparing antilymphocyte globulin induction to no induction in renal transplant patients. Transplantation 2008;86(12):1732‐7. [MEDLINE: ] - PubMed
-
- Cantarovich M, Durrbach A, Ladouceur M, Hiesse C, Benoit G, Charpentier B. 20 year follow‐up results of a randomized controlled trial comparing anti‐lymphocyte globulin‐induction to no induction in renal transplant patients [abstract no: 535]. American Journal of Transplantation 2008;8(Suppl 2):321. - PubMed
-
- Cantarovich M, Durrbach A, Ladouceur M, Hiesse C, Benoit G, Charpentier B. 20 year follow‐up results of a randomized controlled trial comparing anti‐lymphocyte globulin‐induction to no induction in renal transplant patients [abstract no: 879]. Transplantation 2008;86(2S):307. - PubMed
-
- Durrbach A, Cantarovich M, Hiesse C, Benoir G, Charpentier B. Short and long‐term impact of anti‐lymphocyte globulin induction on clinical outcomes in renal transplant patients: 15‐year results of a prospective and randomized trial [abstract no: 2341]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, FL. 2002. [CENTRAL: CN‐00420825]
Charpentier 2002 {published data only}
-
- Charpentier B, European Tacrolimus vs Microemulsified Cyclosporin Study Group. A three arm study comparing immediate tacrolimus therapy with ATG induction therapy followed by either tacrolimus or cyclosporine in adult renal transplant recipients. Transplantation Proceedings 2002;34(5):1625‐6. [MEDLINE: ] - PubMed
-
- Charpentier B, Rostaing L, Berthoux F, Lang P, Civati G, Touraine JL, et al. A three‐arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation 2003;75(6):844‐51. [MEDLINE: ] - PubMed
-
- Rostaing L, Tacrolimus versus Microemulsified Cyclosporin Study Group. Comparison of ATG induction followed by tacrolimus therapy with ATG induction followed by cyclosporine therapy, and immediate tacrolimus‐based triple therapy [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):916A. [CENTRAL: CN‐00433645]
Chatterjee 1976 {published data only}
-
- Chatterjee SN. Antithymocyte globulin in renal transplant recipients. Report of a prospective randomized controlled trial. Archives of Surgery 1976;111(6):680‐3. [MEDLINE: ] - PubMed
Ciancio 2005 {published data only}
-
- Baruqui JA, Ciancio G, Gaynor J, Guerra G, Sageshima J, Chen L, et al. Randomized trial of three induction antibodies in kidney transplantation: long‐term results [abstract no: P‐37]. American Journal of Transplantation 2014;14(Suppl 3):80. [EMBASE: 71388259] - PubMed
-
- Carreno MR, Ciancio G, Burke GW, Rosen A, Ricordi C, Tzakis A, et al. Cellular phenotypes affected by induction therapy with campath‐1h vs thymoglobulin vs zenapax in kidney allograft recipients [abstract]. American Journal of Transplantation 2004;4(Suppl 8):405. [CENTRAL: CN‐00509121]
-
- Ciancio G, Burke GW, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, et al. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune‐monitoring. Transplantation 2005;80(4):457‐65. [MEDLINE: ] - PubMed
-
- Ciancio G, Burke GW, Gaynor JJ, Mattiazzi AD, Carreno MR, Rosen A, et al. Randomized trial of three different induction regimens to prevent acute renal allograft rejection: early results [abstract]. American Journal of Transplantation 2004;4(Suppl 8):266. [CENTRAL: CN‐00509135]
-
- Ciancio G, Burke GW, Gaynor JJ, Roth D, Kupin W, Rosen A, et al. A randomized trial of thymoglobulin vs. alemtuzumab (with lower dose maintenance immunosuppression) vs. daclizumab in renal transplantation at 24 months of follow‐up. Clinical Transplantation 2008;22(2):200‐10. [MEDLINE: ] - PubMed
Ciancio 2010 {published data only}
-
- Baruqui JA, Ciancio G, Gaynor J, Guerra G, Sageshima J, Chen L, et al. Randomized trial of three induction antibodies in kidney transplantation: long‐term results [abstract no: P‐37]. American Journal of Transplantation 2014;14(Suppl 3):80. [EMBASE: 71388259] - PubMed
-
- Ciancio G, Gaynor JJ, Guerra G, Sageshima J, Chen L, Mattiazzi A, et al. Randomized trial of three induction antibodies in kidney transplantation: long‐term results. Transplantation 2014;97(11):1128‐38. [MEDLINE: ] - PubMed
-
- Ciancio G, Gaynor JJ, Roth D, Kupin W, Hanson L, Tueros L, et al. Randomized trial of thymoglobulin versus alemtuzumab (with lower dose maintenance immunosuppression) versus daclizumab in living donor renal transplantation. Transplantation Proceedings 2010;42(9):3503‐6. [MEDLINE: ] - PubMed
Cole 1994 {published data only}
-
- Cole EH, Cattran DC, Farewell VT, Aprile M, Bear RA, Pei YP, et al. A comparison of rabbit antithymocyte serum and OKT3 as prophylaxis against renal allograft rejection. Transplantation 1994;57(1):60‐7. [MEDLINE: ] - PubMed
Condie 1985 {published data only}
-
- Condie RM, Waskosky KE, Hall BL. Efficacy of Minnesota antilymphoblast globulin in renal transplantation: a multicenter, placebo‐controlled, prospective, randomized, double‐blind study. Transplantation Proceedings 1985;17(1 Pt 2):1304‐10. [EMBASE: 1985078723]
Cosimi 1976 {published data only}
-
- Cosimi AB. The clinical value of antilymphocyte antibodies. Transplantation Proceedings 1981;13(1 Pt 1):462‐8. [MEDLINE: ] - PubMed
-
- Cosimi AB, Wortis HH, Delmonico FL, Russell PS. Randomized clinical trial of antithymocyte globulin in cadaver renal allograft recipients: importance of T cell monitoring. Surgery 1976;80(2):155‐63. [MEDLINE: ] - PubMed
-
- Wechter WJ, Brodie JA, Morrell RM, Rafi M, Schultz JR. Antithymocyte globulin (ATGAM) in renal allograft recipients. Multicenter trials using a 14‐dose regimen. Transplantation 1979;28(4):294‐302. [MEDLINE: ] - PubMed
Debure 1987 {published data only}
-
- Debure A, Chkoff N, Chatenoud L, Lacombe M, Campos H, Noel LH, et al. One‐month prophylactic use of OKT3 in cadaver kidney transplant recipients. Transplantation 1988;45(3):546‐53. [MEDLINE: ] - PubMed
-
- Debure A, Chkoff N, Chatenoud L, Lacombe M, Campos H, Noel LH, et al. Preventive treatment of rejection by the prolonged administration of OKT3: decrease of the immune response of the host [Traitement prophylactique du rejet par l'administration prolongee d'OKT3: diminution de la reponse immune de l'hote]. Nephrologie 1987;8(3):87‐94. [MEDLINE: ] - PubMed
De Pauw 1990 {published data only}
-
- Pauw L, Abramowicz D, Goldman M, Vereerstraeten P, Kinnaert P, Toussaint C. Comparison between prophylactic use of OKT3 and cyclosporine in cadaveric renal transplantation. Transplantation Proceedings 1990;22(4):1759‐60. [MEDLINE: ] - PubMed
Diethelm 1979 {published data only}
-
- Diethelm AG, Blackstone E, Whelchel JD, Pass RF, Chambers L, Phillips SJ, et al. The adjunctive value of equine antithymocyte membrane globulin in a randomized study of patients undergoing cadaveric renal transplantation. Transplantation Proceedings 1979;11(1):27‐30. [MEDLINE: ] - PubMed
EARTS Study 1999 {published data only}
-
- Salmela K, Wramner L, Ekberg H, Hauser I, Bentdal O, Lins LE, et al. A randomized multicenter trial of the anti‐ICAM‐1 monoclonal antibody (enlimomab) for the prevention of acute rejection and delayed onset of graft function in cadaveric renal transplantation: a report of the European Anti‐ICAM‐1 Renal Transplant Study Group. Transplantation 1999;67(5):729‐36. [MEDLINE: ] - PubMed
Ejaz 2013 {published data only}
-
- Ejaz N, Shields A, Alloway R, Sadaka B, Girnita A, Mogilishetty G, et al. A prospective, randomized pilot study of B‐cell targeted induction therapy in sensitized kidney transplant recipients: final report [abstract no: 175]. American Journal of Transplantation 2013;13(Suppl S5):84. [EMBASE: 71056751] - PubMed
-
- Ejaz NS, Shields AR, Alloway RR, Sadaka B, Girnita AL, Mogilishetty G, et al. Randomized controlled pilot study of B cell‐targeted induction therapy in HLA sensitized kidney transplant recipients. American Journal of Transplantation 2013;13(12):3142‐54. [MEDLINE: ] - PubMed
-
- Schmidt N, Shields AR, Alloway RR, Sadaka B, Mogilishetty G, Kremer J, et al. Randomized controlled trial of B‐cell targeted induction therapy in HLA sensitized kidney transplant recipients: preliminary results [abstract no: 100]. American Journal of Transplantation 2012;12(Suppl S3):56. [EMBASE: 70746047] - PubMed
Farney 2008 {published data only}
-
- Doares W, Ashcraft E, Singh R, Hart L, Farney A, Hartmann E, et al. Prospective, randomized, single‐center trial of alemtuzumab vs rabbit anti‐thymocyte globulin induction in renal and pancreas transplant: infectious complications update [abstract no: 315]. American Journal of Transplantation 2009;9(Suppl 2):282‐3. [EMBASE: 70010188]
-
- Farney A, Rogers J, Doares W, Iskandar S, Orlando G, Adams P, et al. 7 year results of a prospective randomized study of alemtuzumab vs rabbit anti‐thymocyte globulin induction in kidney and kidney pancreas transplantation [abstract]. Transplantation 2014;98(Suppl 1):90. [EMBASE: 71543817]
-
- Farney A, Rogers J, Hart L, Doares W, Iskandar S, Orlando G, et al. Long‐term results of a prospective randomized study of alemtuzumab vs rabbit anti‐thymocyte globulin induction in kidney and kidney pancreas transplantation [abstract no: 101]. American Journal of Transplantation 2012;12(Suppl S3):56. [EMBASE: 70746048]
-
- Farney A, Singh R, Rogers J, Ashcroft E, Hartmann E, Hart L, et al. A prospective randomized trial of alemtuzumab versus rabbit anti‐thymocyte globulin induction in kidney and pancreas transplantation: minimum 6 months follow up [abstract no: 796]. Transplantation 2008;86(2S):278. [CENTRAL: CN‐00740550]
-
- Farney A, Sundberg A, Moore P, Hartmann E, Rogers J, Doares W, et al. A randomized trial of alemtuzumab vs. anti‐thymocyte globulin induction in renal and pancreas transplantation. Clinical Transplantation 2008;22(1):41‐9. [MEDLINE: ] - PubMed
Frey 1991 {published data only}
-
- Frey DJ, Matas AJ, Gillingham KJ, Canafax D, Payne WD, Dunn DL, et al. MALG vs OKT3 following renal transplantation: a randomized prospective trial. Transplantation Proceedings 1991;23(1 Pt 2):1048‐9. [MEDLINE: ] - PubMed
-
- Frey DJ, Matas AJ, Gillingham KJ, Canafax D, Payne WD, Dunn DL, et al. Sequential therapy‐‐a prospective randomized trial of MALG versus OKT3 for prophylactic immunosuppression in cadaver renal allograft recipients. Transplantation 1992;54(1):50‐6. [MEDLINE: ] - PubMed
Friend 1987 {published data only}
-
- Friend PJ, Calne RY, Hale G, Waldmann H, Evans DB, Rolles K, et al. Prophylactic use of an antilymphocyte monoclonal antibody following renal transplantation: a randomized controlled trial. Transplantation Proceedings 1987;19(1 Pt 3):1898‐900. [MEDLINE: ] - PubMed
-
- Friend PJ, Hale G, Waldmann H, Gore S, Thiru S, Joysey V, et al. Campath‐1M‐‐prophylactic use after kidney transplantation. A randomized controlled clinical trial. Transplantation 1989;48(2):248‐53. [MEDLINE: ] - PubMed
-
- Friend PJ, Waldmann H, Hale G, Tighe H, Calne R. The use of anti‐lymphocyte monoclonal antibodies following organ transplantation [abstract]. Nephrology Dialysis Transplantation 1988;3(1):97‐8. [CENTRAL: CN‐00796675]
Fries 1988a {published data only}
-
- Fries D. Optimal results in cadaver renal transplantation using prophylactic ALG, cyclosporin (CsA) and prednisone (P) [abstract]. Nephrology Dialysis Transplantation 1988;3(1):95. [CENTRAL: CN‐00260355] - PubMed
-
- Fries D, Hiesse C, Charpentier B, Lantz O, Bensadoun H, Benoit G. A single center experience with "low‐dose" cyclosporine in cadaveric renal transplantation. Clinical Transplants 1988:115‐29. [MEDLINE: ] - PubMed
Fukuuchi 1996 {published data only}
-
- Fukuuchi F, Lefrancois N, Bosshard S, Chapuis F, Dubernard JM, Touraine JL. Comparison of prophylactic OKT3 versus ATG in immunologic high risk cadaver renal transplant recipients [abstract]. Journal of the American Society of Nephrology 1995;6(3):1084. [CENTRAL: CN‐00484030]
-
- Fukuuchi F, Lefrancois N, Chapuis F, Gebuhrer L, Bosshard S, Dubernard JM, et al. Comparative efficacy of prophylactic monoclonal (OKT3) and polyclonal antibodies (ATG) in immunologic high‐risk renal transplant recipients. Transplantation Proceedings 1996;28(5):2808‐9. [MEDLINE: ] - PubMed
Gianello 1987 {published data only}
-
- Gianello P, Squifflet JP, Pirson Y, Stoffel M, Dereme T, Alexandre GP. Cyclosporine‐steroids versus conventional therapy in cadaver kidney transplantation: analysis of a randomized trial at two years. Transplantation Proceedings 1987;19(1 Pt 3):1867‐72. [MEDLINE: ] - PubMed
Grafals 2014 {published data only}
-
- Grafals M, Simpson M, Gilligan H, Pomposelli J, Akoad M, Kwaja K, et al. Prospective randomized study of low dose antithymocyte globulin as induction in non sensitized adult renal transplant recipients [abstract no: C1351]. American Journal of Transplantation 2013;13(Suppl 5):430. [EMBASE: 71057927]
-
- Smith B, Grafals M, Murakami N, Trabucco A, Hamill K, Marangos E, et al. Immune phenotyping and efficacy of low dose ATG in non‐sensitized kidney recipients undergoing early steroid withdrawal: a randomized pilot study [abstract]. Transplantation 2014;98(Suppl 1):580. [EMBASE: 71545491] - PMC - PubMed
Grino 1990 {published data only}
-
- Grino JM, Alsina J, Sabater R, Castelao AM, Gil‐Vernet S, Andres E, et al. Antilymphoblast globulin, cyclosporine, and steroids in cadaveric renal transplantation. Transplantation 1990;49(6):1114‐7. [2360253] - PubMed
-
- Koga A, Moreso FJ, Seron D, Gil‐Vernet S, Cruzado JM, Castelao AM, et al. Beneficial effect of concomitant induction with antilymphoblast globulin, cyclosporine, and steroids on long‐term renal allograft outcome. Transplantation Proceedings 2004;36(5):1305‐7. [MEDLINE: ] - PubMed
Grino 1991 {published data only}
-
- Gonzalez C, Grino JM, Castelao AM, Seron D, Gil‐Vernet S, Andres E, et al. Pre‐transplant ALG, low dose cyclosporine (CsA) and steroids versus pre‐transplant OKT3, CsA and steroids in kidney cadaveric transplantation [abstract]. Kidney International 1990;37(6):1601. [CENTRAL: CN‐00601919]
-
- Grino JM, Castelao AM, Gonzalez C, Seron D, Gil‐Vernet S, Andres E, et al. Pre‐transplant ALG, low dose cyclosporine (CYA) and steroids in kidney cadaveric transplantation [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:513A. [CENTRAL: CN‐00601920]
-
- Grino JM, Castelao AM, Seron D, Gonzalez C, Galceran JM, Gil‐Vernet S, et al. Antilymphocyte globulin versus OKT3 induction therapy in cadaveric kidney transplantation: a prospective randomized study. American Journal of Kidney Diseases 1992;20(6):603‐10. [MEDLINE: ] - PubMed
-
- Grino JM, Castelao AM, Seron D, Gonzalez C, Galceran JM, Gil‐Vernet S, et al. Prophylactic OKT3, CyA, and steroids versus antilymphoblast globulin, CyA, and steroids in cadaveric kidney transplantation. Transplantation Proceedings 1992;24(1):39‐41. [MEDLINE: ] - PubMed
-
- Grino JM, Castelao AM, Seron D, Gonzalez C, Gil‐Vernet S, Andres E, et al. Antilymphocyte serum, cyclosporine and corticoids, versus OKT3, cyclosporine, and corticoids in kidney transplantation [Serum anti‐lymphocyte, ciclosporine et corticoides, versus OKT3, ciclosporine et corticoides en transplantation renale]. Presse Medicale 1991;20(40):2039‐42. [MEDLINE: ] - PubMed
Grundmann 1984 {published data only}
-
- Grundmann R. Infectious diseases under prophylactic ALG treatment and their prevention in a prospectively randomized trial. Scandinavian Journal of Urology & Nephrology Supplementum 1985;92:33‐5. [MEDLINE: ] - PubMed
-
- Grundmann R, Wienand P, Hesse U. Improvement in cyclosporine handling by anti‐lymphocyte globulin in the early post‐operative period. Proceedings of the European Dialysis & Transplant Association ‐ European Renal Association 1985;21:987‐91. [MEDLINE: ] - PubMed
-
- Grundmann R, Wienand P, Hesse U. Improvement of the cyclosporin A handling by ALG in the early postoperative period [abstract]. Kidney International 1984;26(4):637. [CENTRAL: CN‐00601926]
-
- Grundmann R, Wienand P, Meider G, Vlaho V, Pichlmaier H. Use and limits of preventive antilymphocyte globulin therapy following kidney transplantation. A prospective randomized study [Nutzen und grenzen einer prophylaktischen antilymphozytenglobulin‐therapie nach nierentransplantation. Eine prospektiv randomisierte studie]. Klinische Wochenschrift 1984;62(20):979‐85. [MEDLINE: ] - PubMed
Grundmann 1987 {published data only}
-
- Grundmann R, Hesse U, Wienand P, Baldamus C, Arns W. Graft survival and long‐term renal function after sequential conventional cyclosporin A therapy in cadaver kidney transplantation‐‐a prospective randomized trial. Klinische Wochenschrift 1987;65(18):879‐84. [MEDLINE: ] - PubMed
-
- Grundmann R, Wienand P, Hesse U. Sequential conventional and cyclosporine therapy in cadaver renal transplantation‐‐a prospective randomized trial. Transplantation Proceedings 1987;19(5):4033‐4. [MEDLINE: ] - PubMed
Guttmann 1997 {published data only}
-
- Guttmann RD, TEAMS1 Group (Transplant European Antilfa Multicenter Study Group). Randomized clinical trial of anti‐lfa‐1 monoclonal antibody in cadaveric renal transplantation: a European multicenter study [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:258. [CENTRAL: CN‐00509222]
Halloran 1982 {published data only}
-
- Halloran P, Ludwin D, Aprile M. Randomized comparison between cyclosporine and conventional therapy plus Minnesota antilymphocyte globulin in cadaveric renal transplantation. Transplantation Proceedings 1983;15(4 Suppl 1‐2):2513‐6. [EMBASE: 1984089086]
-
- Halloran PF, Lien J, Aprile M, White N. Preliminary results of a randomized comparison of cyclosporine and Minnesota antilymphoblast globulin. Transplantation Proceedings 1982;14(4):627‐30. [MEDLINE: ] - PubMed
Hanaway 2011 {published data only}
-
- Hanaway M, Woodle ES, Mulgaonkar S, Peddi R, Harrison G, Vandeputte K, et al. 12 month results of a multicenter, randomized trial comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 135]. American Journal of Transplantation 2008;8(Suppl 2):215.
-
- Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, et al. Alemtuzumab induction in renal transplantation. New England Journal of Medicine 2011;364(20):1909‐19. [MEDLINE: ] - PubMed
-
- Holman J, Harrison G, Vandeputte K, First R, Fitzsimmons W. Immune cell activation comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 553]. Transplantation 2008;86(2 Suppl):194. [CENTRAL: CN‐00676047]
-
- Mulgaonkar S, Hanaway M, Woodle ES, Peddi R, Harrison G, Vandeputte K, et al. Continuing 24 month results of a multicenter, randomized trial comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and a rapid steroid withdrawal in renal transplantation [abstract no: 312]. American Journal of Transplantation 2009;9(Suppl 2):282. [EMBASE: 70010185]
-
- Peddi R, Hanaway M, Woodle S, Mulgaonkar S, Harrison G, Vandeputte K, et al. Final 36 month results of a randomized trial comparing three induction agents (alemtuzumab, thymoglobulin and basiliximab) with tacrolimus, mycophenolate mofetil and rapid steroid withdrawal in renal transplantation [abstract no: 35]. American Journal of Transplantation 2010;10(Suppl 4):49. [EMBASE: 70463396]
Hanto 1991 {published data only}
-
- Hanto DW, Jendrisak MD, McCullough CS, So SK, Marsh JW, Rush T, et al. A prospective randomized comparison of prophylactic ALG and OKT3 in cadaver kidney allograft recipients. Transplantation Proceedings 1991;23(1 Pt 2):1050‐1. [MEDLINE: ] - PubMed
-
- Hanto DW, Jendrisak MD, So SK, McCullough CS, Rush TM, Michalski SM, et al. Induction immunosuppression with antilymphocyte globulin or OKT3 in cadaver kidney transplantation. Results of a single institution prospective randomized trial. Transplantation 1994;57(3):377‐84. [MEDLINE: ] - PubMed
Henry 2001 {published data only}
-
- Henry ML, Elkhammas EA, Bumgardner G, Davies EA, Pelletier RP, et al. A prospective randomized trial of neoral and cellcept with and without OKT3 induction [abstract]. Transplantation 1998;65(12):S191. [CENTRAL: CN‐00445692]
-
- Henry ML, Pelletier RP, Elkhammas EA, Bumgardner GL, Davies EA, Ferguson RM. A randomized prospective trial of OKT3 induction in the current immunosuppression era. Clinical Transplantation 2001;15(6):410‐4. [MEDLINE: ] - PubMed
Hourmant 1985a {published data only}
-
- Hourmant M, Soulillou JP, Guenel J. Comparison of three immuno‐suppressive regimens in kidney transplantation: CyA, ATG and CyA, ATG and conventional treatment ‐ a one‐center randomized study [abstract]. Kidney International 1984;26(4):639. [CENTRAL: CN‐00626072]
-
- Hourmant M, Soulillou JP, Guenel J. Comparison of three immunosuppressive regimens in kidney transplantation: a single‐centre randomised study. Proceedings of the European Dialysis & Transplant Association ‐ European Renal Association 1985;21:982‐6. [MEDLINE: ] - PubMed
-
- Hourmant M, Soulillou JP, Guenel J. Comparison of three immunosuppressive strategies in kidney transplantation: Antithymocyte globulin and conventional treatment, antithymocyte globulin and cyclosporine, and cyclosporine. A one‐center randomized study. Transplantation Proceedings 1985;17(1 Pt 2):1158‐61. [EMBASE: 1985079646]
Hourmant 1996 {published data only}
-
- Hourmant M. Multicenter comparative study of an anti‐lfa1 adhesion molecule monoclonal antibody and thymoglobulin in prophylaxis of acute rejection in kidney transplantation. [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid, Spain. 1995:336. [CENTRAL: CN‐00509239]
-
- Hourmant M, Bedrossian J, Durand D, Kessler M, Branchu Y, Caudrelier P, et al. Multicenter comparative study of an anti‐LFA‐1 adhesion molecule monoclonal antibody and antithymocyte globulin in prophylaxis of acute rejection in kidney transplantation. Transplantation Proceedings 1995;27(1):864. [MEDLINE: ] - PubMed
-
- Hourmant M, Bedrossian J, Durand D, Kessler M, Lebranchu Y, Caudrelier P, et al. Multicenter study of an anti‐LFA1 adhesion molecule monoclonal antibody (Moab) and anti‐thymocyte globulin (ATG) in prophylaxis of acute rejection (AR) in kidney transplantation (KT) [abstract]. 14th Annual Meeting. American Society of Transplant Physicians (ASTP); 1995 May 14‐17; Chicago (ILL). 1995.
-
- Hourmant M, Bedrossian J, Durand D, Lebranchu Y, Renoult E, Caudrelier P, et al. A randomized multicenter trial comparing leukocyte function‐associated antigen‐1 monoclonal antibody with rabbit antithymocyte globulin as induction treatment in first kidney transplantations. Transplantation 1996;62(11):1565‐70. [MEDLINE: ] - PubMed
Jakobsen 1981 {published data only}
-
- Jakobsen A, Flatmark A, Lundgren G, Solheim B, Groth CG. A controlled trial with AHLG Behring: lack of effect on cadaveric renal graft survival. Scandinavian Journal of Urology & Nephrology Supplementum 1981;64:205‐12. [MEDLINE: ] - PubMed
Kasiske 1997 {published data only}
-
- Kasiske BL, Goerdt PJ, Heim‐Duthoy K, Rao KV, Dahl DC, Ney AL, et al. Interim results of a randomized controlled trial comparing antithymocyte globulin (ATG) with cyclosporine (CSA) induction [abstract]. Journal of the American Society of Nephrology 1995;6(3):1097. [CENTRAL: CN‐00484599]
-
- Kasiske BL, Johnson HJ, Goerdt PJ, Heim‐Duthoy KL, Rao VK, Dahl DC, et al. A randomized trial comparing cyclosporine induction with sequential therapy in renal transplant recipients. American Journal of Kidney Diseases 1997;30(5):639‐45. [MEDLINE: ] - PubMed
Khosroshahi 2008 {published data only}
-
- Khosroshahi HT, Tubbs RS, Shoja MM, Ghafari A, Noshad H, Ardalan MR. Effect of prophylaxis with low‐dose anti‐thymocyte globulin on prevention of acute kidney allograft rejection. Transplantation Proceedings 2008;40(1):137‐9. [MEDLINE: ] - PubMed
Kountz 1977 {published data only}
-
- Butt KM, Zielinski CM, Parsa I, Elberg AJ, Wechter W, Kountz SL. Trends in immunosuppression for kidney transplantation. Kidney International ‐ Supplement 1978;13(Suppl 8):S95‐8. [MEDLINE: ] - PubMed
-
- Kountz SL, Butt KH, Rao TK, Zielinski CM, Rafi M, Schultz JR. Antithymocyte globulin (ATG) dosage and graft survival in renal transplantation. Transplantation Proceedings 1977;9(1):1023‐5. [MEDLINE: ] - PubMed
Kreis 1980 {published data only}
-
- Crosnier J, Kreis H, Descamps JM, Mansouri R. Are there non‐steroid‐dependent rejection episodes?. Proceedings of the European Dialysis & Transplant Association 1980;17:391‐5. [MEDLINE: ] - PubMed
-
- Kreis H, Mansouri R, Descamps JM, Dandavino R, N'Guyen AT, Bach JF, et al. Antithymocyte globulin in cadaver kidney transplantation: a randomized trial based on T‐cell monitoring. Kidney International 1981;19(3):438‐44. [MEDLINE: ] - PubMed
Kreis 1986 {published data only}
-
- Kreis H, Chkoff N, Chatenoud L. Prolonged administration of a monoclonal anti‐T3 cell antibody (ORTHOCLONE OKT3) to kidney allograft recipients. Transplantation Proceedings 1986;18(4):954‐6. [EMBASE: 1986223864]
Kumar 1998a {published data only}
-
- Kumar MS, Cahill K, Kumar AM, Panigrahi D, Seirka D, Singleton R, et al. ATGAM versus OKT3 induction therapy in cadaveric kidney transplantation: patient and graft survival, CD3 subset, infection, and cost analysis. Transplantation Proceedings 1998;30(4):1351‐2. [MEDLINE: ] - PubMed
-
- Kumar MS, Laskow DA, Panigrahi D, Kumar AM, Cahill K, Pankewyez O, et al. Correlation of CD3 counts and serum IL‐10 levels during induction to incidence of rejections and infections in cadaveric kidney recipients [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):683A. [CENTRAL: CN‐00446225]
Launois 1977 {published data only}
-
- Launois B, Campion JP, Fauchet R, Kerbaol M, Cartier F. Prospective randomized clinical trial in patients with cadaver‐kidney transplants. Transplantation Proceedings 1977;9(1):1027‐30. [MEDLINE: ] - PubMed
Lazarovits 1993 {published data only}
-
- Lazarovits AI, Rochon J, Banks L, Hollomby DJ, Muirhead N, Jevnikar AM, et al. Human mouse chimeric CD7 monoclonal antibody (SDZCHH380) for the prophylaxis of kidney transplant rejection. Journal of Immunology 1993;150(11):5163‐74. [MEDLINE: ] - PubMed
-
- Lazarovits AI, Rochon J, Banks L, Hollomby DJ, Muirhead N, Jevnikar AM, et al. Human mouse chimeric CD7 monoclonal antibody (SDZCHH380) for the prophylaxis of kidney transplant rejection. Transplantation Proceedings 1993;25(1 Pt 1):820‐2. [MEDLINE: ] - PubMed
-
- Sharma L, Muirhead N, Lazarovits AI. Human mouse chimeric Cd7 monoclonal antibody (SCZCHH380) for the prophylaxis of kidney transplant rejection: no transplant losses and absence of chronic rejection at 4 years [abstract]. 15th Annual Meeting American Society of Transplant Physicians (ASTP); 1996 May 10‐14; Chicago Ill. 1996.
Lu 2011 {published data only}
-
- Lu TM, Yang SL, Wu WZ, Tan JM. Alemtuzumab induction therapy in highly sensitized kidney transplant recipients. Chinese Medical Journal 2011;124(5):664‐8. [MEDLINE: ] - PubMed
Maiorca 1984 {published data only}
-
- Maiorca R, Cristinelli L, Scolari S, Sandrini S, Brunori G, Tonini G, et al. Prospective controlled trial with antilynfocytic globulin (A.L.G.), in first cadaveric renal transplants treated with low‐dose steroids both in prophylaxis and rejection therapy [abstract]. Kidney International 1984;26(4):644. [CENTRAL: CN‐00775915]
Margreiter 2008 {published data only}
-
- Margreiter R, Klempnauer J, Neuhaus P, Muehlbacher F. Alemtuzumab (Campath‐1H) induction followed by tacrolimus monotherapy vs tacrolimus based triple drug immunosuppression in cadaveric renal transplantation ‐ results of a multicenter trial [abstract no: 333]. American Journal of Transplantation 2007;7(Suppl 2):234. [CENTRAL: CN‐00644175]
-
- Margreiter R, Klempnauer J, Neuhaus P, Muehlbacher F, Boesmueller C, Calne RY. Alemtuzumab (Campath‐1H) and tacrolimus monotherapy after renal transplantation: results of a prospective randomized trial. American Journal of Transplantation 2008;8(7):1480‐5. [MEDLINE: ] - PubMed
Martins 2004 {published data only}
-
- Martins LS, Sarmento AM, Dias L, Henriques AC, Borner G, Cabrita A. Results of safety and efficacy of two ATG bolus‐therapy regimen vs standard therapy in prophylaxis after renal transplantation in end stage renal disease patients [abstract]. Transplantation 2004;78(2 Suppl):266. [CENTRAL: CN‐00509341]
Michael 1989 {published data only}
-
- Michael H, Francos G, Burke J, Besarab A, Jarrell B, Moritz M, et al. Effect of cyclosporine (CYA) versus antilymphocyte globulin (ALG) on delayed graft function (DGF) in renal transplant patients [abstract]. Kidney International 1989;35(1):520. [CENTRAL: CN‐00626110] - PubMed
-
- Michael HJ, Francos GC, Burke JF, Besarab A, Moritz M, Gillum D, et al. A comparison of the effects of cyclosporine versus antilymphocyte globulin on delayed graft function in cadaver renal transplant recipients. Transplantation 1989;48(5):805‐8. [MEDLINE: ] - PubMed
Minnesota Study 1982 {published data only}
-
- Boudreaux JP, McHugh L, Canafax DM, Ascher N, Sutherland DE, Payne W, et al. The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation 1987;44(3):376‐81. [MEDLINE: ] - PubMed
-
- Canafax DM, Min DI, Gruber SA, Matas AJ, Payne WD, Dunn DL, et al. Immunosuppression for cadaveric renal allograft recipients: a risk‐factor matched comparison of the Minnesota randomized trial with an antilymphoblast globulin, azathioprine, cyclosporine, and prednisone protocol. Clinical Transplantation 1989;3(2):110‐9. [EMBASE: 1989138098]
-
- Canafax DM, Simmons RL, Sutherland DE, Fryd DS, Strand MH, Ascher NL, et al. Early and late effects of two immunosuppressive drug protocols on recipients of renal allografts: results of the Minnesota randomized trial comparing cyclosporine versus antilymphocyte globulin‐azathioprine. Transplantation Proceedings 1986;18(2 Suppl 1):192‐6. [MEDLINE: ] - PubMed
-
- Canafax DM, Torres A, Fryd DS, Heil JE, Strand MH, Ascher NL, et al. The effects of delayed function on recipients of cadaver renal allografts. A study of 158 patients randomized to cyclosporine or ALG‐azathioprine. Transplantation 1986;41(2):177‐81. [MEDLINE: ] - PubMed
-
- Ferguson RM, Rynasiewicz JJ, Sutherland DE, Simmons RL, Najarian JS. Cyclosporin A in renal transplantation: a prospective randomized trial. Surgery 1982;92(2):175‐82. [MEDLINE: ] - PubMed
Morales 1994a {published data only}
-
- Anonymous. Cyclosporine monotherapy versus OKT3 and cyclosporine versus prednisone and cyclosporine as induction therapy in older renal transplant patients: a multicenter randomized study. Spanish Monotherapy Study Group. Transplantation Proceedings 1994;26(5):2522‐4. [MEDLINE: ] - PubMed
-
- Morales JM, Marcen R, Grino JM, Arias M, Vilardell J. Comparison of three induction protocols in older renal transplant patients: cyclosporine (CYA) monotherapy (M) vs OKT3 and CYNA vs prednisone (P) and CYA. A multicenter randomized study [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):950. [CENTRAL: CN‐00485149]
Mourad 1998 {published data only}
-
- Anonymous. Tacrolimus in renal transplantation: a comparison of induction vs noninduction therapy (triple therapy): three‐month results. Transplantation Proceedings 1999;31(1‐2):330‐1. [MEDLINE: ] - PubMed
-
- Antoine C, Thakur S, Daugas E, Fraoui R, Boudjeltia S, Julia P, et al. Vascular microthrombosis in renal transplant recipients treated with tacrolimus. Transplantation Proceedings 1998;30(6):2813‐4. [MEDLINE: ] - PubMed
-
- Charpentier B. An induction versus no‐induction protocol in anticalcineurin‐based immunosuppression using very low‐dose steroids. Transplantation Proceedings 2001;33(4 Suppl):3S‐10S. [MEDLINE: ] - PubMed
-
- Charpentier B. Induction versus non‐induction protocols in anti‐calcineurin‐based immunosuppression. Transplantation Proceedings 2001;33(7‐8):3334‐6. [MEDLINE: ] - PubMed
-
- Mourad G, French and Belgian Tacrolimus Renal Transplantation Study Group. ATG‐Induction vs. non‐induction with tacrolimus‐based immunosuppression in renal transplantation [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome, Italy. 2000. [CENTRAL: CN‐00446848]
Niaudet 1990 {published data only}
-
- Niaudet P, Murcia I, Jean G, Broyer M. A comparative trial of OKT3 and antilymphocyte serum in the preventive treatment of rejection after kidney transplantation in children [Essai comparatif de l'OKT3 et du serum antilymphocytaire dans le traitement preventif du rejet apres transplantation renale chez l'enfant]. Annales de Pediatrie 1990;37(2):83‐5. [MEDLINE: ] - PubMed
Norman 1988 {published data only}
-
- Norman DJ, Shield CF 3rd, Barry J, Bennett WM, Henell K, Kimball J, et al. Early use of OKT3 monoclonal antibody in renal transplantation to prevent rejection. American Journal of Kidney Diseases 1988;11(2):107‐10. [MEDLINE: ] - PubMed
Norman 1993 {published data only}
-
- Abramowicz D, Norman DJ, Goldman M, Pauw L, Kinnaert P, Kahana L, et al. OKT3 prophylaxis improves long‐term renal graft survival in high‐risk patients as compared to cyclosporine: combined results from the prospective, randomized Belgian and US studies. Transplantation Proceedings 1995;27(1):852‐3. [MEDLINE: ] - PubMed
-
- Abramowicz D, Norman DJ, Vereerstraeten P, Goldman M, Pauw L, Vanherweghem JL, et al. OKT3 prophylaxis in renal grafts with prolonged cold ischemia times: association with improvement in long‐term survival. Kidney International 1996;49(3):768‐72. [MEDLINE: ] - PubMed
-
- Norman DJ, Kahana L, Stuart FP Jr, Thistlethwaite JR Jr, Shield CF 3rd, Monaco A, et al. A randomized clinical trial of induction therapy with OKT3 in kidney transplantation. Transplantation 1993;55(1):44‐50. [MEDLINE: ] - PubMed
-
- Shield CF 3rd, Jacobs RJ, Wyant S, Das A. A cost‐effectiveness analysis of OKT3 induction therapy in cadaveric kidney transplantation. American Journal of Kidney Diseases 1996;27(6):855‐64. [MEDLINE: ] - PubMed
Norman 1993a {published data only}
-
- Norman DJ, Kimball JA, Barry JM. Cytokine‐release syndrome: differences between high and low doses of OKT3. Transplantation Proceedings 1993;25(2 Suppl 1):35‐8. [MEDLINE: ] - PubMed
-
- Norman DJ, Kimball JA, Bennett WM, Shihab F, Batiuk TD, Meyer MM, et al. A prospective, double‐blind, randomized study of high‐versus low‐dose OKT3 induction immunosuppression in cadaveric renal transplantation. Transplant International 1994;7(5):356‐61. [MEDLINE: ] - PubMed
Norrby 1997 {published data only}
-
- Norrby J, Olausson M. A randomized clinical trial using ATG Fresenius or ATG Merieux as induction therapy in kidney transplantation. Transplantation Proceedings 1997;29(7):3135‐6. [MEDLINE: ] - PubMed
Novick 1983 {published data only}
-
- Novick AC, Braun WE, Steinmuller D, Buszta C, Greenstreet R, Kiser W. A controlled randomized double‐blind study of antilymphoblast globulin in cadaver renal transplantation. Transplantation 1983;35(2):175‐9. [MEDLINE: ] - PubMed
Perez‐Tamajon 1996 {published data only}
-
- Perez‐Tamajon L, González JM, Torres A, Rodriguez A, Hernandez D, Losada M, et al. Induction treatment with sequential quadruple therapy in renal transplantation: polyclonal vs. monoclonal AC at low doses [abstract]. Nefrología 1996;16:97. [CENTRAL: CN‐00602023]
Pernin 2012 {published data only}
-
- Pernin V, Portales P, Szwarc I, Garrigue V, Vetromile F, Delmas S, et al. Lymphocyte reconstitution after induction with thymoglobulin in renal transplantation: Impact of the mode of administration (daily vs intermittent treatment based on T lymphocyte monitoring) [abstract no: SAP671]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii533‐4. [EMBASE: 70766907]
Raffaele 1991 {published data only}
-
- Raffaele P, Pouteil‐Noble C, Lefrancois N, Bosshard S, Betuel H, Aymard M, et al. Influence of a randomized monoclonal or polyclonal program of therapy on cytomegalovirus infection in kidney transplantation. Transplantation Proceedings 1991;23(1 Pt 2):1361‐2. [MEDLINE: ] - PubMed
Rostaing 2010 {published data only}
-
- Rostaing L, Lavayssiere L, Kamar N. Hematologic adverse effects of 2 different polyclonal antilymphocyte preparations in de novo kidney transplant patients. Experimental & Clinical Transplantation 2010;8(2):178‐80. [MEDLINE: ] - PubMed
Sakhrani 1992 {published data only}
-
- Sakhrani L, Aswad S, Obispo E, Mendez RG, Khetan U, Asai P, et al. Optimal dose of Minnesota antilymphocyte globulin for induction immunosuppression in cadaveric renal transplants. Transplantation Proceedings 1992;24(5):1730‐1. [MEDLINE: ] - PubMed
Samsel 1999 {published data only}
-
- Samsel R, Chmura A, Korczak‐Kowalska G, Wlodarczyk Z, Pliszczynski J, Adadynski L, et al. Long term results of single perioperative high dose of ATG as induction in kidney transplantation [abstract]. American Journal of Transplantation 2004;4(Suppl 8):351. [CENTRAL: CN‐00509456]
-
- Samsel R, Chmura A, Wlodarczyk Z, Wyzgal J, Cieciura T, Lagiewska B, et al. Perioperative single high dose ATG‐Fresenius S administration as induction immunosuppressive therapy in cadaveric renal transplantation‐‐preliminary results. Annals of Transplantation 1999;4(2):37‐9. [MEDLINE: ] - PubMed
-
- Samsel R, Pliszczynski J, Chmura A, Korczak G, Wlodarczyk Z, Cieciura T, et al. Safety and efficacy of high dose ATG bolus administration on rewascularization in kidney graft patients‐‐long term results. Annals of Transplantation 2008;13(1):32‐9. [MEDLINE: ] - PubMed
-
- Samsel R, Rowinski W, Chmura A, Wlodarczyk Z, Wyzgal J, Cieciura T, et al. Perioperative administration of single, high‐dose of ATG‐Fresenius‐S as an induction immunosuppressive therapy in cadaveric renal transplantation: preliminary results. Transplantation Proceedings 2001;33(6):2952‐4. [MEDLINE: ] - PubMed
Sansom 1976 {published data only}
-
- Sansom JR, Barnes AD, Hall CL. A randomized prospective clinical trial of antilymphocyte globulin in 100 cadaveric renal transplants. Postgraduate Medical Journal 1976;52(5 Suppl):75‐8. [MEDLINE: ] - PubMed
Sharaf El Din 2006 {published data only}
-
- Sharaf EI Din UA, Sharaf EI Din BA, EI Damanhoury HA. Alemtuzumab induction as steroid sparing agent in living kidney transplantation: a randomized prospective controlled trial [abstract no: SA‐PO449]. Journal of the American Society of Nephrology 2006;17(Abstracts):670A. [CENTRAL: CN‐00644281]
Sheashaa 2008 {published data only}
-
- Sheashaa HA, Hamdy AF, Bakr MA, Abdelbaset SF, Ghoneim MA. Long‐term evaluation of single bolus high dose ATG induction therapy for prophylaxis of rejection in live donor kidney transplantation. International Urology & Nephrology 2008;40(2):515‐20. [MEDLINE: ] - PubMed
Shield 1993 {published data only}
-
- Shield CF 3rd, Beilman G. Safety of OKT3 use in the operating room. Transplantation Proceedings 1993;25(2 Suppl 1):43‐4. [MEDLINE: ] - PubMed
Slakey 1993 {published data only}
-
- Johnson CP, Slakey DP, Callaluce RD, Browne BJ, Roza AM, Adams MB. Prospective randomized comparison of quadruple vs triple therapy for first cadaver transplants with immediate function. Transplantation Proceedings 1993;25(1 Pt 1):585‐6. [MEDLINE: ] - PubMed
-
- Slakey DP, Johnson CP, Callaluce RD, Browne BJ, Zhu YR, Roza AM, et al. A prospective randomized comparison of quadruple versus triple therapy for first cadaver transplants with immediate function. Transplantation 1993;56(4):827‐31. [MEDLINE: ] - PubMed
Smeekens 2013 {published data only}
-
- Joosten I, Baas MC, Kamburova EG, Hoogen MW, Koenen HJ, Hilbrands LB. Anti‐B cell therapy with rituximab as induction therapy in renal transplantation. Transplant Immunology 2014;31(4):207‐9. [MEDLINE: ] - PubMed
-
- Kamburova E, Koenen H, Hoogen M, Joosten I, Hilbrands L. A single dose of rituximab results in a long lasting B‐cell depletion in peripheral blood, without affecting the peripheral T‐cell compartment [abstract no: 186]. American Journal of Transplantation 2013;13(Suppl S5):87. [EMBASE: 71056762]
-
- Kamburova EG, Hoogen MW, Koenen HJ, Baas MC, Hilbrands LB, Joosten I. Cytokine release after treatment with rituximab in renal transplant recipients. Transplantation 2015;99(9):1907‐11. [MEDLINE: ] - PubMed
-
- Smeekens SP, Hoogen MW, Kamburova EG, Veerdonk FL, Joosten I, Koenen HJ, et al. The effects of in vivo B‐cell depleting therapy on ex‐vivo cytokine production. Transplant Immunology 2013;28(4):183‐8. [MEDLINE: ] - PubMed
Spillner 1998 {published data only}
-
- Spillner J, Kohnle M, Albrecht KH, Heemann U. Anti‐LFA‐1 monoclonal antibody in renal transplantation: renal function, infections, and other complications. Transplantation Proceedings 1998;30(5):2163. [MEDLINE: ] - PubMed
Squifflet 1997 {published data only}
-
- Squifflet JP, Besse T, Malaise J, Mourad M, Delcorde C, Hope JA, et al. BTI‐322 for induction therapy after renal transplantation: a randomized study. Transplantation Proceedings 1997;29(1‐2):317‐9. [MEDLINE: ] - PubMed
-
- Squifflet JP, Besse T, Mourad M, Malaise J, Delcorde C, Pirson Y, et al. A randomized study of BTI‐322 for the prevention of renal allograft rejection: one year follow up [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:258. [CENTRAL: CN‐00509490]
Steinmuller 1991 {published data only}
-
- Steinmuller DR, Hayes J, Novick AC, Streem SB, Hodge E, Slavis S, et al. Comparison of OKT3 to ALG for prophylaxis for patients with acute renal failure after cadaver renal transplantation [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:512A. [CENTRAL: CN‐00716029] - PubMed
-
- Steinmuller DR, Hayes JM, Novick AC, Streem SB, Hodge E, Slavis S, et al. Comparison of OKT3 with ALG for prophylaxis for patients with acute renal failure after cadaveric renal transplantation. Transplantation 1991;52(1):67‐71. [MEDLINE: ] - PubMed
Stevens 2008 {published data only}
-
- Stevens R, Foster K, Miles C, Rigley T, Kalil A, Wrenshall L. A randomized 2X2 factorial trial: Part 1, single‐dose rATG induction improves long‐term renal transplant outcomes [abstract]. Transplantation 2014;98(Suppl 1):91. [EMBASE: 71543822]
-
- Stevens R, Foster K, Miles C, Rigley T, Wrenshall L. A randomized trial of renal transplantation: Steroid and calcineurin‐inhibitor withdrawal after rabbit anti‐thymocyte globulin induction [abstract]. Transplantation 2014;98(Suppl 1):476. [EMBASE: 71545124]
-
- Stevens RB. Modern approaches to combining sirolimus with calcineurin inhibitors. Transplantation Proceedings 2008;40(10 Suppl):S21‐4. [MEDLINE: ] - PubMed
-
- Stevens RB, Foster KW, Miles CD, Kalil AC, Florescu DF, Sandoz JP, et al. A randomized 2x2 factorial clinical trial of renal transplantation: steroid‐free maintenance immunosuppression with calcineurin inhibitor withdrawal after six months associates with improved renal function and reduced chronic histopathology. PLoS ONE [Electronic Resource] 2015;10(10):e0139247. [MEDLINE: ] - PMC - PubMed
Taylor 1976 {published data only}
-
- Ackman CF, Taylor HE, Schual RS. Long‐term follow up of Canadian clinical trial of antilymphocyte globulin in renal transplantation [abstract]. Kidney International 1979;15(6):709. [CENTRAL: CN‐00444100]
-
- MacDonald AS, Belitsky P, Lannon SG, Cohen A, White J. Antithymocyte globulin in cadaver renal grafts: prophylaxis and antirejection therapy with three different preparations. Transplantation Proceedings 1982;14(4):631‐4. [MEDLINE: ] - PubMed
Thibaudin 1998 {published data only}
-
- Thibaudin D, Alamartine E, Filippis JP, Diab N, Laurent B, Berthoux F. Advantage of antithymocyte globulin induction in sensitized kidney recipients: a randomized prospective study comparing induction with and without antithymocyte globulin. Nephrology Dialysis Transplantation 1998;13(3):711‐5. [MEDLINE: ] - PubMed
Thomas 1977 {published data only}
-
- Thomas F, Mendez‐Picon G, Thomas J, Peace K, Flora R, Lee HM. Effect of antilyphocyte‐globulin potency on survival of cadaver renal transplants. Prospective randomised double‐blind trial. Lancet 1977;2(8040):671‐4. [MEDLINE: ] - PubMed
Thomas 2007 {published data only}
-
- Thomas PG, Woodside KJ, Lappin JA, Vaidya S, Rajaraman S, Gugliuzza KK. Alemtuzumab (Campath 1H) induction with tacrolimus monotherapy is safe for high immunological risk Renal transplantation. Transplantation 2007;83(11):1509‐12. [MEDLINE: ] - PubMed
Toledo‐Pereyra 1985 {published data only}
-
- Toledo‐Pereyra LH, Bergren C, Mittal VK, Whitten JI, Baskin S, McNichol L. A prospective randomized comparison of antilymphoblast globulin versus antithymocyte globulin for cadaver kidney transplantation. Transplantation 1985;40(4):448‐50. [MEDLINE: ] - PubMed
-
- Toledo‐Pereyra LH, Bergren C, Whitten J. Comparison of ALG and ATG for renal transplantation [abstract]. Kidney International 1985;28(2):388. [CENTRAL: CN‐00583280]
TRIMS Study 2010 {published data only}
-
- Woodle ES, Peddi VR, Tomlanovich S, Mulgaonkar S, Kuo PC, TRIMS Study Investigators. A prospective, randomized, multicenter study evaluating early corticosteroid withdrawal with thymoglobulin in living‐donor kidney transplantation. Clinical Transplantation 2010;24(1):73‐83. [MEDLINE: ] - PubMed
-
- Woodle ES, TRIMS Study Group. A randomized, prospective, multicenter comparative study evaluating a thymoglobulin‐based early corticosteroid cessation regime in renal transplantation [abstract no: 673]. American Journal of Transplantation 2006;6(Suppl 2):294. [CENTRAL: CN‐00716028]
-
- Woodle ES, TRIMS Study Group. A randomized, prospective, multicenter study of thymoglobulin in renal transplantation for induction and minimization of steroids (TRIMS) [abstract no: 1632]. American Journal of Transplantation 2005;5(Suppl 11):571. [CENTRAL: CN‐00716027]
Tsai 2012 {published data only}
-
- Tsai MK, Lee CY, Yang CY, Yeh CC, Hu RH, Lee PH. Rituximab induction therapy provided additional immunosuppressive effect and functional benefit to non‐sensitized renal transplant recipients: an interim report [abstract no: 997]. American Journal of Transplantation 2012;12(Suppl S3):319. [EMBASE: 70746950]
Turcotte 1973 {published data only}
-
- Turcotte JG, Feduska NJ, Haines RF, Freier DT, Gikas PW, McDonald FD, et al. Antithymocyte globulin in renal transplant recipients. A clinical trial. Archives of Surgery 1973;106(4):484‐8. [MEDLINE: ] - PubMed
Tyden 2009 {published data only}
-
- Tyden G, Genberg H, Tollemar J, Ekberg H, Persson NH, Tufveson G, et al. A randomized, doubleblind, placebo‐controlled, study of single‐dose rituximab as induction in renal transplantation. Transplantation 2009;87(9):1325‐9. [MEDLINE: ] - PubMed
-
- Tyden G, Mjornstedt L, Ekberg H, Tufveson G. A prospective, randomised, placebo controlled, multicenter study of the efficacy and safety of rituximab as induction therapy together with tacrolimus, mycophenolate mofetil and steroids in renal transplantation [abstract no: 861]. Transplantation 2008;86(2S):300. [CENTRAL: CN‐00763744]
-
- Tyden G, Mjornstedt L, Ekberg H, Tufveson G. Is rituximab safe to use in kidney transplant patients?. American Journal of Transplantation 2010;10(8):1949. [MEDLINE: ] - PubMed
-
- Tydén G, Ekberg H, Tufveson G, Mjörnstedt L. A randomized, double‐blind, placebo‐controlled study of single dose rituximab as induction in renal transplantation: a 3‐year follow‐up. Transplantation. 2012;94(3):e21‐2. [MEDLINE: ] - PubMed
van den Hoogen 2013 {published data only}
-
- Hoogen MW, Kho MM, Abrahams AC, Zuilen AD, Sanders JS, Dijk M, et al. Effect of a single intraoperative high‐dose ATG‐Fresenius on delayed graft function in donation after cardiac‐death donor renal allograft recipients: a randomized study. Experimental & Clinical Transplantation 2013;11(2):134‐41. [MEDLINE: ] - PubMed
Vela 1994 {published data only}
-
- Vela C, Cristol JP, Chong G, Okamba A, Lorho R, Mion C, et al. Antilymphocyte globulins versus OKT3 as prophylactic treatment in highly sensitized renal transplant recipients. Transplant International 1994;7 Suppl 1:S259‐62. [MEDLINE: ] - PubMed
Vigeral 1986 {published data only}
-
- Vigeral P, Chkoff N, Chatenoud L, Campos H, Lacombe M, Droz D, et al. Prophylactic use of OKT3 monoclonal antibody in cadaver kidney recipients. Utilization of OKT3 as the sole immunosuppressive agent. Transplantation 1986;41(6):730‐3. [MEDLINE: ] - PubMed
Wechter 1979 {published data only}
-
- Wechter WJ, Morrell RM, Bergan J, Rosenberg JC, Turcotte J, Schultz JR. Extended treatment with antithymocyte globulin (ATGAM) in renal allograft recipients. Transplantation 1979;28(5):365‐7. [MEDLINE: ] - PubMed
Yussim 2000 {published data only}
-
- Yussim A, Shapira Z. Single‐bolus high‐dose ATG for prophylaxis of rejection in renal transplantation‐‐a prospective, randomized study. Transplant International 2000;13 Suppl 1:S293‐4. [MEDLINE: ] - PubMed
References to studies excluded from this review
Alloway 1993 {published data only}
-
- Alloway R, Kotb M, Hathaway D, Ohman M, Strain S, Gaber AO. The pharmacokinetic profile of standard and low‐dose OKT3 induction immunosuppression in renal transplant recipients. Transplantation 1994;58(2):249‐53. [MEDLINE: ] - PubMed
-
- Alloway R, Kotb M, Hathaway DK, Gaber LW, Vera SR, Gaber AO. Randomized double‐blind study of standard versus low‐dose OKT3 induction therapy in renal allograft recipients. American Journal of Kidney Diseases 1993;22(1):36‐43. [MEDLINE: ] - PubMed
-
- Alloway R, Kotb M, Hathaway DK, Gaber LW, Vera SR, Gaber AO. Results of a prospective, randomized double‐blind study comparing standard vs low‐dose OKT3 induction therapy. Transplantation Proceedings 1993;25(1 Pt 1):550‐2. [MEDLINE: ] - PubMed
-
- Chaballa M, Alloway RR, Kotb M, Hathaway DK, Gaber L, Vera SR, et al. Five year follow up of prospective, randomized double‐blind study comparing standard versus low dose OKT3 induction therapy in renal allograft recipients [abstract]. 16th Annual Meeting. American Society of Transplant Physicians (ASTP); 1997 May 10‐14; Chicago (ILL). 1997:259. [CENTRAL: CN‐00509127]
Kirsch 2006 {published data only}
-
- Kirsch BM, Haidinger M, Zeyda M, Bohmig GA, Tombinsky J, Muhlbacher F, et al. Alemtuzumab (Campath‐1H) induction therapy and dendritic cells: impact on peripheral dendritic cell repertoire in renal allograft recipients. Transplant Immunology 2006;16(3‐4):254‐7. [MEDLINE: ] - PubMed
Kumar 2002b {published data only}
-
- Kumar A, Zaman W, Chaurasia D, Gupta A, Sharma RK, Gulati S. Prospective randomized trial to evaluate the efficacy of single low dose ATG induction in renal transplant recipient with spousal kidney. Indian Journal of Urology 2002;19(1):58‐62. [EMBASE: 36347895]
NCT00000936 {unpublished data only}
-
- Blechman‐Krom I. Controlled trial of induction therapy in renal transplantation. www.clinicaltrials.gov/ct2/show/NCT00000936 (accessed 3 November 2016).
NCT01312064 {unpublished data only}
-
- Tsai MK. Clinical outcome of de novo everolimus‐based immunosuppressive therapy for renal transplantation using rituximab induction. www.clinicaltrials.gov/ct2/show/NCT01312064 (accessed 22 August 2016).
References to studies awaiting assessment
NCT00089947 {published data only}
-
- NCT00089947. Randomized, prospective, phase 2 study comparing thymoglobulin in a rapid discontinuation of corticosteroids protocol with standard corticosteroid therapy in living donor renal transplantation using mycophenolate mofetil and tacrolimus maintenance therapy. clinicaltrials.gov/ct2/show/NCT00089947 (accessed 22 August 2016).
NCT00861536 {published data only}
-
- Steiger J. An open, multicenter, randomised, parallel group pilot study to investigate two different polyclonal rabbit immunoglobulin preparations for safety and efficacy: a comparison of ATG‐fresenius to thymoglobulin in prophylaxis for immunological high risk patients following renal transplantation. www.clinicaltrials gov/ct2/show/NCT00861536 (accessed 22 August 2016).
NCT01046955 {unpublished data only}
-
- Burke GW. Head‐to‐head comparison of thymoglobulin vs. campath‐1h vs. our standard center treatment protocol in living donor renal transplantation ‐ a study to evaluate the avoidance of long‐term nephrotoxic calcineurin inhibitor therapy. www.clinicaltrials.gov/ct2/show/NCT01046955 (accessed 3 November 2016).
NCT01354301 {unpublished data only}
-
- Tedesco H. Efficacy and safety of induction strategies combined with low tacrolimus exposure in kidney transplant recipients receiving everolimus or sodium mycophenolate. www.clinicaltrials.gov/ct2/show/NCT01354301 (accessed 3 November 2016).
Stevens 2016 {published data only}
-
- Stevens RB, Wrenshall LE, Miles CD, Farney AC, Jie T, Sandoz JP, et al. A double‐blind, double‐dummy, flexible‐design randomized multicenter trial: early safety of single‐ versus divided‐dose rabbit anti‐thymocyte globulin induction in renal transplantation. American Journal of Transplantation 2016;16(6):1858‐67. [MEDLINE: ] - PMC - PubMed
References to ongoing studies
NCT00733733 {published data only}
-
- Hoitsma AJ. A prospective, randomized, open, multicenter study to evaluate the efficacy and tolerability of induction therapy with a single high‐dose anti‐t‐lymphocyte globulin (ATG) in renal transplant patients with a kidney from a non‐heart‐beating donor and tacrolimus, mycophenolate mofetil, and steroids as basic immunosuppression. www.clinicaltrials.gov/ct2/show/NCT00733733 (accessed 22 August 2016).
NCT01154387 {unpublished data only}
-
- Flechner S. A two part, phase 1/2, safety, PK and PD study of TOL101, an anti‐TCR monoclonal antibody for prophylaxis of acute organ rejection in patients receiving renal transplantation. www.clinicaltrials.gov/ct2/show/NCT01154387 (accessed 22 August 2016).
ReMIND Study 2013 {unpublished data only}
-
- Mamode M. A randomized trial of rituximab in induction therapy for living donor renal transplantation. www.clinicaltrials.gov/ct2/show/NCT01095172 (accessed 22 August 2016).
Additional references
ANZDATA 2009
-
- ANZDATA. [direct communication]. Australian and New Zealand Dialysis and Transplant Registry 2009.
Calne 1999
-
- Calne R, Moffatt SD, Friend PJ, Jamieson NV, Bradley JA, Hale G, et al. Campath IH allows low‐dose cyclosporine monotherapy in 31 cadaveric renal allograft recipients. Transplantation 1999;68(10):613‐6. [MEDLINE: ] - PubMed
Cibrik 2001
-
- Cibrik DM, Kaplan B, Meier‐Kriesche HU. Role of anti‐interleukin‐2 receptor antibodies in kidney transplantation. Biodrugs 2001;15(10):655‐66. [MEDLINE: ] - PubMed
Denton 1999
-
- Denton MD, Magee CC, Sayegh MH. Immunosuppressive strategies in transplantation. Lancet 1999;353(9158):1083‐91. [MEDLINE: ] - PubMed
GRADE 2008
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated February 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hong 2000
-
- Hong JC, Kahan BD. Sirolimus‐induced thrombocytopenia and leukopenia in renal transplant recipients: risk factors, incidence, progression, and management. Transplantation 2000;69(10):2085‐90. [MEDLINE: ] - PubMed
KDIGO 2009
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation 2009;9 Suppl 3:S1‐155. [MEDLINE: ] - PubMed
Kreis 1992
-
- Kreis H. Antilymphocyte globulins in kidney transplantation. Kidney International ‐ Supplement 1992;38:188‐92. [MEDLINE: ] - PubMed
Meier‐Kriesche 2004
-
- Meier‐Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. American Journal of Transplantation 2004;4(3):378‐83. [MEDLINE: ] - PubMed
Racusen 1999
-
- Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney international 1999;55(2):713‐23. [MEDLINE: ] - PubMed
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Solez 2007
-
- Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, et al. Banff '05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy ('CAN'). American Journal of Transplantation 2007; Vol. 7, issue 3:518‐26. [MEDLINE: ] - PubMed
Soulillou 2001
-
- Soulillou JP, Giral M. Controlling the incidence of infection and malignancy by modifying immunosuppression. Transplantation 2001;72(12 Suppl):S89‐93. [MEDLINE: ] - PubMed
Tonelli 2011
-
- Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. American Journal of Transplantation 2011;11(10):2093‐109. [MEDLINE: ] - PubMed
Tyden 2003
-
- Tyden G, Kumlien G, Fehrman I. Successful ABO‐incompatible kidney transplantations without splenectomy using antigen specific immunoadsorption and rituximab. Transplantation 2003;76(4):730‐1. [MEDLINE: ] - PubMed
UNOS 2011
-
- United Network for Organ Sharing. OPTN data [direct communication]. www.unos.org (accessed 4 November 2016).
Webster 2006
Webster 2010
Zand 2007
-
- Zand MS. Therapeutic antibody agents for B‐cell immunomodulation in renal transplantation. Transplantation 2007;84(11 S Suppl):S11‐9. [EMBASE: 350294707]
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical