A novel method for the quantification of fatty infiltration in skeletal muscle
- PMID: 28073372
- PMCID: PMC5223468
- DOI: 10.1186/s13395-016-0118-2
A novel method for the quantification of fatty infiltration in skeletal muscle
Abstract
Background: Fatty infiltration of the skeletal muscle is a common but poorly understood feature of many myopathies. It is best described in human muscle, where non-invasive imaging techniques and representative histology have been optimized to view and quantify infiltrating fat. However, human studies are limited in their ability to identify cellular and molecular mechanisms regulating fatty infiltration, a likely prerequisite to developing targeted interventions. As mechanistic investigations move to small animals, studies may benefit from new or adapted imaging tools optimized for high resolution and whole muscle quantification.
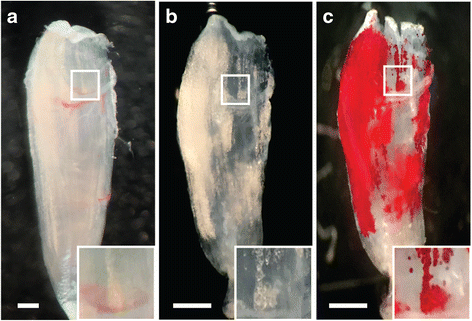
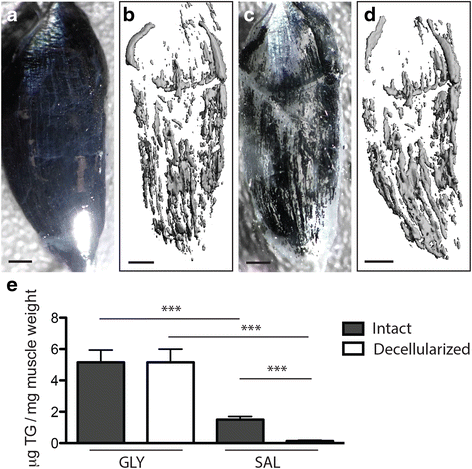
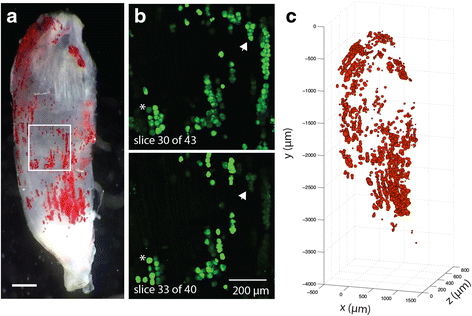
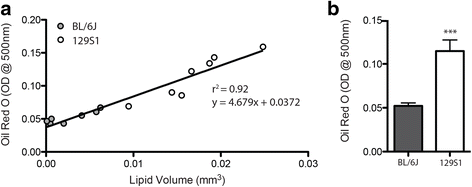
Results: Here, we describe a novel method to evaluate fatty infiltration, developed for use with mouse muscle. In this methodology, muscle cellular membranes and proteins are removed via decellularization, but fatty infiltrate lipid is spared, trapped in its native distribution in a transparent extracellular matrix construct. This lipid can then be stained with visible or fluorescent dyes and imaged. We present three methods to stain and evaluate lipid in decellularized muscles which can be used individually or combined: (1) qualitative visualization of the amount and 3D spatial distribution of fatty infiltration using visible lipid soluble dye Oil Red O (ORO), (2) quantitative analysis of individual lipid droplet metrics (e.g., volume) via confocal imaging of fluorescent lipid soluble dye boron-dipyrromethene (BODIPY), and (3) quantitative analysis of total lipid content by optical density reading of extracted stained lipid. This methodology was validated by comparing glycerol-induced fatty infiltration between two commonly used mouse strains: 129S1/SvlmJ (129S1) and C57BL/6J (BL/6J). All three methods were able to detect a significant increase in fatty infiltrate volume in the 129S1 muscle compared with that in BL/6J, and methods 1 and 2 additionally described a difference in the distribution of fatty infiltrate, indicating susceptibility to glycerol-induced fatty infiltration is strain-specific.
Conclusions: With more mechanistic studies of fatty infiltration moving to small animal models, having an alternative to expensive non-invasive imaging techniques and selective representative histology will be beneficial. In this work, we present a method that can quantify both individual adipocyte lipids and whole muscle total fatty infiltrate lipid.
Keywords: Fatty infiltration; IMAT; Intramuscular fat; Muscle lipid.
Figures


References
-
- Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. - PubMed
-
- Cheema B, Abas H, Smith B, O'Sullivan AJ, Chan M, Patwardhan A, et al. Investigation of skeletal muscle quantity and quality in end-stage renal disease. Nephrology (Carlton) Blackwell publ asia. 2010;15:454–463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

