Tau passive immunization inhibits not only tau but also Aβ pathology
- PMID: 28073379
- PMCID: PMC5225540
- DOI: 10.1186/s13195-016-0227-5
Tau passive immunization inhibits not only tau but also Aβ pathology
Abstract
Background: Accumulation of hyperphosphorylated tau protein is a histopathological hallmark of Alzheimer's disease (AD) and related tauopathies. Currently, there is no effective treatment available for these progressive neurodegenerative diseases. In recent years, tau immunotherapy has shown great potential in animal models. We report the effect of immunization with tau antibodies 43D against tau 6-18 and 77E9 against tau 184-195 on tau and amyloid-β (Aβ) pathologies and cognition in triple-transgenic (3×Tg)-AD mice at mild to moderate stages of the disease.
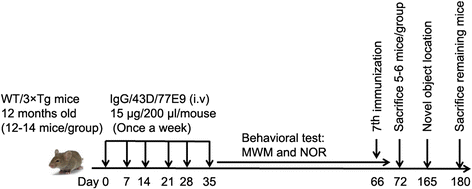
Methods: We immunized 12-month-old female 3×Tg-AD mice with two to six or seven intravenous weekly doses of 15 μg of mouse monoclonal antibody 43D, 77E9, a combination of one-half dose each of 43D and 77E9, or as control of mouse immunoglobulin G (IgG). Age-matched wild-type mice treated with mouse IgG or a mixture of 43D and 77E9 were also used as controls. The effect of immunization with tau antibodies on tau and Aβ pathologies was assessed by Western blot and immunofluorescence analysis, and the effect on cognition was analyzed by using Morris water maze, one-trial novel object recognition, and novel object location tasks.
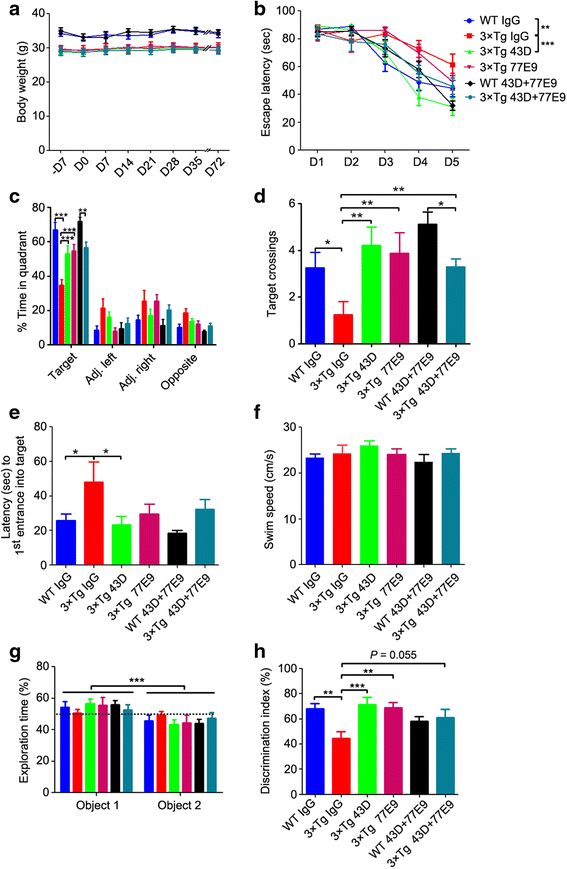
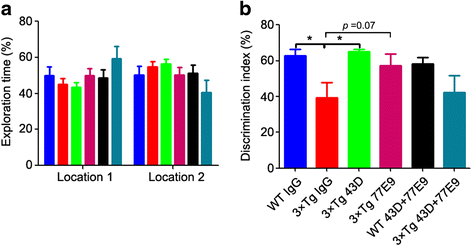
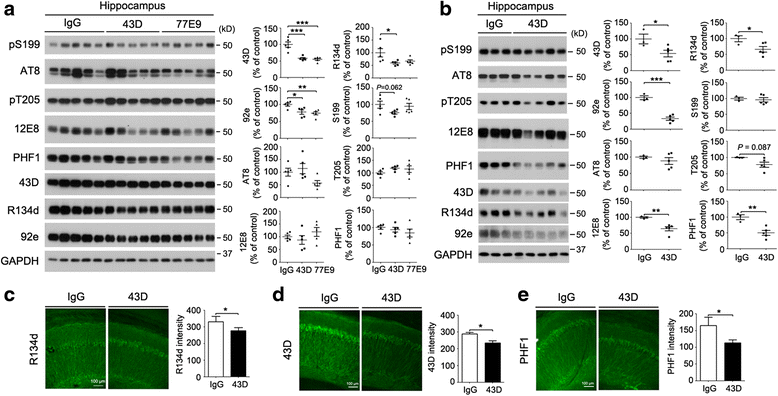
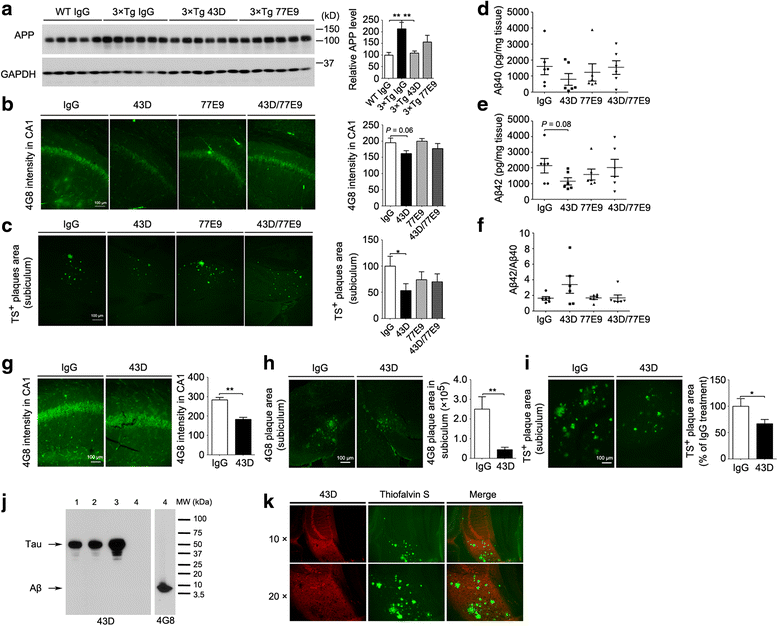
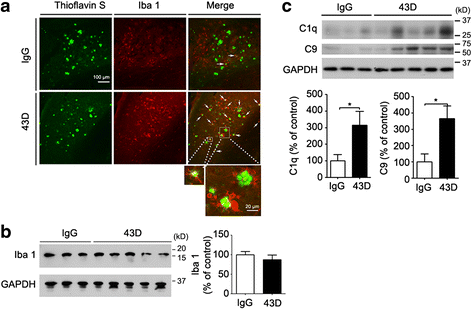
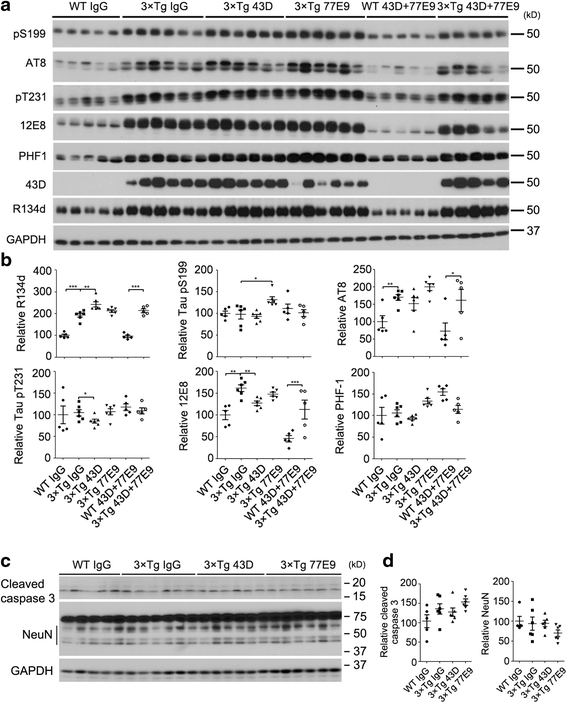
Results: We found that two doses of 43D and 77E9 reduced total tau but had no significant impact on hyperphosphorylation of tau. However, six doses of 43D reduced levels of both total tau and tau hyperphosphorylated at Ser262/356 and Ser396/404 sites in the hippocampus. Importantly, both 43D and 77E9 antibodies rescued spatial memory and short-term memory impairments in 3×Tg-AD mice. The beneficial effect of 43D and 77E9 antibodies on cognitive performance was sustained up to 3 months after the last dose. Six doses of immunization with 43D also decreased amyloid precursor protein (APP) level in CA1 and amyloid plaques in subiculum, and showed a trend toward reducing Aβ40 and Aβ42 in the forebrain. Immunization with 43D increased levels of complement components C1 and C9 and resulted in activation of microglia, especially surrounding Aβ plaques.
Conclusions: These findings suggest the potential of passive immunization targeting proximal N-terminal domain tau 6-18 as a disease-modifying approach to AD and related tauopathies.
Keywords: Alzheimer’s disease; Amyloid-β; Immunotherapy; Tau; Tauopathy.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

