Design, synthesis and biological evaluation of regioisomers of 666-15 as inhibitors of CREB-mediated gene transcription
- PMID: 28073675
- PMCID: PMC5296214
- DOI: 10.1016/j.bmcl.2016.12.078
Design, synthesis and biological evaluation of regioisomers of 666-15 as inhibitors of CREB-mediated gene transcription
Abstract
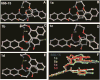
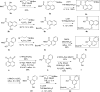
cAMP-response element binding protein (CREB) is a nuclear transcription factor that has been implicated in the pathogenesis and maintenance of various types of human cancers. Identification of small molecule inhibitors of CREB-mediated gene transcription has been pursued as a novel strategy for developing cancer therapeutics. We recently discovered a potent and cell-permeable CREB inhibitor called 666-15. 666-15 is a bisnaphthamide and has been shown to possess efficacious anti-breast cancer activity without toxicity in vivo. In this study, we designed and synthesized a series of analogs of 666-15 to probe the importance of regiochemistry in naphthalene ring B. Biological evaluations of these analogs demonstrated that the substitution pattern of the alkoxy and carboxamide in naphthalene ring B is very critical for maintaining potent CREB inhibition activity, suggesting that the unique bioactive conformation accessible in 666-15 is critically important.
Keywords: 666-15; Bioactive conformation; CREB; Cancer; Regioisomer.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Discovery of a Synergistic Inhibitor of cAMP-Response Element Binding Protein (CREB)-Mediated Gene Transcription with 666-15.J Med Chem. 2019 Dec 26;62(24):11423-11429. doi: 10.1021/acs.jmedchem.9b01207. Epub 2019 Dec 10. J Med Chem. 2019. PMID: 31765143 Free PMC article.
-
Identification of a Potent Inhibitor of CREB-Mediated Gene Transcription with Efficacious in Vivo Anticancer Activity.J Med Chem. 2015 Jun 25;58(12):5075-87. doi: 10.1021/acs.jmedchem.5b00468. Epub 2015 Jun 5. J Med Chem. 2015. PMID: 26023867 Free PMC article.
-
Mechanistic insights into the activation of ester prodrugs of 666-15.Bioorg Med Chem Lett. 2020 Oct 1;30(19):127455. doi: 10.1016/j.bmcl.2020.127455. Epub 2020 Jul 28. Bioorg Med Chem Lett. 2020. PMID: 32730943 Free PMC article.
-
Regulation of CREB-mediated gene expression by salt inducible kinase.J Steroid Biochem Mol Biol. 2008 Feb;108(3-5):287-91. doi: 10.1016/j.jsbmb.2007.09.006. Epub 2007 Sep 7. J Steroid Biochem Mol Biol. 2008. PMID: 17935972 Review.
-
Regulation of somatostatin gene transcription by cyclic adenosine monophosphate.Metabolism. 1996 Aug;45(8 Suppl 1):4-7. doi: 10.1016/s0026-0495(96)90068-2. Metabolism. 1996. PMID: 8769368 Review.
Cited by
-
Naringin Mediates Adult Hippocampal Neurogenesis for Antidepression via Activating CREB Signaling.Front Cell Dev Biol. 2022 Apr 7;10:731831. doi: 10.3389/fcell.2022.731831. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35478969 Free PMC article.
-
SS18-SSX drives CREB activation in synovial sarcoma.Cell Oncol (Dordr). 2022 Jun;45(3):399-413. doi: 10.1007/s13402-022-00673-w. Epub 2022 May 12. Cell Oncol (Dordr). 2022. PMID: 35556229 Free PMC article.
-
Cholesterol metabolism regulated by CAMKK2-CREB signaling promotes castration-resistant prostate cancer.Cell Rep. 2025 Jun 24;44(6):115792. doi: 10.1016/j.celrep.2025.115792. Epub 2025 Jun 7. Cell Rep. 2025. PMID: 40483692 Free PMC article.
-
What turns CREB on? And off? And why does it matter?Cell Mol Life Sci. 2020 Oct;77(20):4049-4067. doi: 10.1007/s00018-020-03525-8. Epub 2020 Apr 28. Cell Mol Life Sci. 2020. PMID: 32347317 Free PMC article. Review.
-
Antiallergic drug desloratadine as a selective antagonist of 5HT2A receptor ameliorates pathology of Alzheimer's disease model mice by improving microglial dysfunction.Aging Cell. 2021 Jan;20(1):e13286. doi: 10.1111/acel.13286. Epub 2020 Dec 24. Aging Cell. 2021. PMID: 33369003 Free PMC article.
References
-
- Lackner MR, Wilson TR, Settleman J. Future Oncol. 2012;8:999–1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

