Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism
- PMID: 28073783
- PMCID: PMC5823234
- DOI: 10.1182/blood-2016-06-720714
Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism
Abstract
Venous thromboembolism (VTE) is common in patients with brain tumors, and underlying mechanisms are unclear. We hypothesized that podoplanin, a sialomucin-like glycoprotein, increases the risk of VTE in primary brain tumors via its ability to induce platelet aggregation. Immunohistochemical staining against podoplanin and intratumoral platelet aggregates was performed in brain tumor specimens of 213 patients (mostly high-grade gliomas [89%]) included in the Vienna Cancer and Thrombosis Study, a prospective observational cohort study of patients with newly diagnosed cancer or progressive disease aimed at identifying patients at risk of VTE. Platelet aggregation in response to primary human glioblastoma cells was investigated in vitro. During 2-year follow-up, 29 (13.6%) patients developed VTE. One-hundred fifty-one tumor specimens stained positive for podoplanin (33 high expression, 47 medium expression, 71 low expression). Patients with podoplanin-positive tumors had lower peripheral blood platelet counts (P < .001) and higher D-dimer levels (P < .001). Podoplanin staining intensity was associated with increasing levels of intravascular platelet aggregates in tumor specimens (P < .001). High podoplanin expression was associated with an increased risk of VTE (hazard ratio for high vs no podoplanin expression: 5.71; 95% confidence interval, 1.52-21.26; P =010), independent of age, sex, and tumor type. Podoplanin-positive primary glioblastoma cells induced aggregation of human platelets in vitro, which could be abrogated by an antipodoplanin antibody. In conclusion, high podoplanin expression in primary brain tumors induces platelet aggregation, correlates with hypercoagulability, and is associated with increased risk of VTE. Our data indicate novel insights into the pathogenesis of VTE in primary brain tumors.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
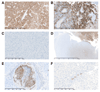
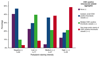


Comment in
-
The tip of the thrombos-is-berg.Sci Transl Med. 2017 Feb 1;9(375):eaam6053. doi: 10.1126/scitranslmed.aam6053. Epub 2017 Feb 1. Sci Transl Med. 2017. PMID: 28148844
-
Risking thromboembolism: podoplanin and glioma.Blood. 2017 Mar 30;129(13):1742-1743. doi: 10.1182/blood-2017-02-763524. Blood. 2017. PMID: 28360357 No abstract available.
References
-
- Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–1723. - PubMed
-
- Semrad TJ, O’Donnell R, Wun T, et al. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg. 2007;106(4):601–608. - PubMed
-
- Jo JT, Schiff D, Perry JR. Thrombosis in brain tumors. Semin Thromb Hemost. 2014;40(3):325–331. - PubMed
-
- Brandes AA, Scelzi E, Salmistraro G, et al. Incidence of risk of thromboembolism during treatment high-grade gliomas: a prospective study. Eur J Cancer. 1997;33(10):1592–1596. - PubMed
-
- Rodas RA, Fenstermaker RA, McKeever PE, et al. Correlation of intraluminal thrombosis in brain tumor vessels with postoperative thrombotic complications: a preliminary report. J Neurosurg. 1998;89(2):200–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

