Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice
- PMID: 28073784
- PMCID: PMC5345735
- DOI: 10.1182/blood-2016-09-741298
Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice
Erratum in
-
McDonald B, Davis RP, Kim S-J, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129(10):1357-1367.Blood. 2022 Feb 10;139(6):952. doi: 10.1182/blood.2021014436. Blood. 2022. PMID: 35142853 Free PMC article. No abstract available.
Abstract
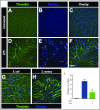
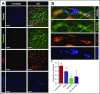
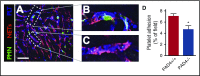
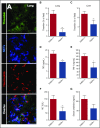
Neutrophil extracellular traps (NETs; webs of DNA coated in antimicrobial proteins) are released into the vasculature during sepsis where they contribute to host defense, but also cause tissue damage and organ dysfunction. Various components of NETs have also been implicated as activators of coagulation. Using multicolor confocal intravital microscopy in mouse models of sepsis, we observed profound platelet aggregation, thrombin activation, and fibrin clot formation within (and downstream of) NETs in vivo. NETs were critical for the development of sepsis-induced intravascular coagulation regardless of the inciting bacterial stimulus (gram-negative, gram-positive, or bacterial products). Removal of NETs via DNase infusion, or in peptidylarginine deiminase-4-deficient mice (which have impaired NET production), resulted in significantly lower quantities of intravascular thrombin activity, reduced platelet aggregation, and improved microvascular perfusion. NET-induced intravascular coagulation was dependent on a collaborative interaction between histone H4 in NETs, platelets, and the release of inorganic polyphosphate. Real-time perfusion imaging revealed markedly improved microvascular perfusion in response to the blockade of NET-induced coagulation, which correlated with reduced markers of systemic intravascular coagulation and end-organ damage in septic mice. Together, these data demonstrate, for the first time in an in vivo model of infection, a dynamic NET-platelet-thrombin axis that promotes intravascular coagulation and microvascular dysfunction in sepsis.
© 2017 by The American Society of Hematology.
Figures



Comment in
-
Sepsis: NET-induced coagulation induces organ damage in sepsis.Nat Rev Nephrol. 2017 Mar;13(3):133. doi: 10.1038/nrneph.2017.7. Epub 2017 Jan 31. Nat Rev Nephrol. 2017. PMID: 28138127 No abstract available.
References
-
- Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244-1250. - PubMed
-
- Dempfle C-E. Coagulopathy of sepsis. Thromb Haemost. 2004;91(2):213-224. - PubMed
-
- Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13(4):463-469. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

