Extended Treatment with Single-Agent Ibrutinib at the 420 mg Dose Leads to Durable Responses in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
- PMID: 28073846
- PMCID: PMC6317338
- DOI: 10.1158/1078-0432.CCR-16-1431
Extended Treatment with Single-Agent Ibrutinib at the 420 mg Dose Leads to Durable Responses in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
Abstract
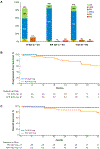
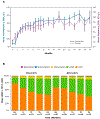
Purpose: Ibrutinib, a first-in-class, once-daily, oral inhibitor of Bruton tyrosine kinase, promotes apoptosis, and inhibits B-cell proliferation, adhesion, and migration. Ibrutinib has demonstrated single-agent efficacy and acceptable tolerability at doses of 420 and 840 mg in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who were treatment-naïve (TN) or had relapsed/refractory (R/R) CLL after ≥1 prior therapy in a phase Ib/II study (PCYC-1102). Subsequently, the ibrutinib 420 mg dose was approved in CLL.Experimental Design: We report data with 44 months of follow-up on 94 patients with TN and R/R CLL/SLL receiving ibrutinib 420 mg once-daily in PCYC-1102 and the long-term extension study PCYC-1103.Results: Ninety-four CLL/SLL patients (27 TN, 67 R/R) were treated with ibrutinib (420 mg/day). Patients with R/R disease had received a median of four prior therapies (range, 1-12). Responses were rapid and durable and median duration of response was not reached. Best overall response was 91% [85% TN (complete response, CR 26%) and 94% R/R (9% CR)]. Median progression-free survival (PFS) was not reached in either group. The 30-month PFS rate was 96% and 76% for TN and R/R patients, respectively. Ibrutinib was well tolerated with extended follow-up; rates of grade ≥3 cytopenias and fatigue, as well as discontinuations due to toxicities decreased over time.Conclusions: Single-agent ibrutinib at 420 mg once-daily resulted in durable responses and was well tolerated with up to 44 months follow-up in patients with TN and R/R CLL/SLL. Currently, 66% of patients continue on ibrutinib. Clin Cancer Res; 23(5); 1149-55. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures

References
-
- Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk Lymphoma 2013;54:2385–91 - PubMed
-
- de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 2012;119:2590–4 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

