Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal
- PMID: 28073888
- PMCID: PMC5561383
- DOI: 10.1136/gutjnl-2016-312473
Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal
Abstract
Background/aims: We studied the role of lysyl oxidase-like 2 (LOXL2) in collagen crosslinking and hepatic progenitor cell (HPC) differentiation, and the therapeutic efficacy of a LOXL2-blocking monoclonal antibody on liver fibrosis progression/reversal in mice.
Methods: Anti-LOXL2 antibody, control antilysyl oxidase antibody or placebo was administered during thioacetamide (TAA)-induced fibrosis progression or during recovery. Therapeutic efficacy in biliary fibrosis was tested in BALB/c.Mdr2-/- and 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)-fed mice. Collagen crosslinking, fibrosis progression and reversal were assessed histologically and biochemically. HPC differentiation was studied in primary EpCAM(+) liver cells in vitro.
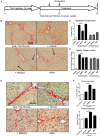
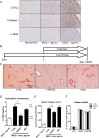
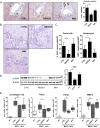
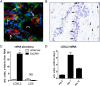
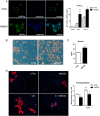
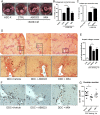
Results: LOXL2 was virtually absent from healthy but strongly induced in fibrotic liver, with predominant localisation within fibrotic septa. Delayed anti-LOXL2 treatment of active TAA fibrosis significantly reduced collagen crosslinking and histological signs of bridging fibrosis, with a 53% reduction in morphometric collagen deposition. In established TAA fibrosis, LOXL2 inhibition promoted fibrosis reversal, with enhanced splitting and thinning of fibrotic septa, and a 45% decrease in collagen area at 4 weeks of recovery. In the Mdr2-/- and DDC-induced models of biliary fibrosis, anti-LOXL2 antibody similarly achieved significant antifibrotic efficacy and suppressed the ductular reaction, while hepatocyte replication increased. Blocking LOXL2 had a profound direct effect on primary EpCAM(+) HPC behaviour in vitro, promoting their differentiation towards hepatocytes, while inhibiting ductal cell lineage commitment.
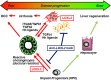
Conclusions: LOXL2 mediates collagen crosslinking and fibrotic matrix stabilisation during liver fibrosis, and independently promotes fibrogenic HPC differentiation. By blocking these two convergent profibrotic pathways, therapeutic LOXL2 inhibition attenuates both parenchymal and biliary fibrosis and promotes fibrosis reversal.
Keywords: CIRRHOSIS; HEPATIC FIBROSIS; HEPATITIS; PRIMARY BILIARY CIRRHOSIS; PRIMARY SCLEROSING CHOLANGITIS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: AM-V and VS are employees of Gilead Sciences which develops anti-LOXL2 antibody and holds related patents. YVP received research funding from Gilead Sciences.
Figures

Comment in
-
Selective LOXL2 inhibition: potent antifibrotic effects in ongoing fibrosis and fibrosis regression.Gut. 2017 Sep;66(9):1540-1541. doi: 10.1136/gutjnl-2016-313621. Epub 2017 Feb 28. Gut. 2017. PMID: 28246311 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
