Management of Malignant Pleural Effusion with ASEPT® Pleural Catheter: Quality of Life, Feasibility, and Patient Satisfaction
- PMID: 28074083
- PMCID: PMC5198149
- DOI: 10.1155/2016/4273480
Management of Malignant Pleural Effusion with ASEPT® Pleural Catheter: Quality of Life, Feasibility, and Patient Satisfaction
Abstract
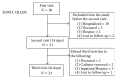
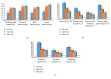
Objective. The PleurX® IPC system has been used extensively in the past. Over time, management of MPE with the PleurX system can be costly. The new ASEPT pleural catheter, through advantages in design, may ultimately show cost savings. The primary outcome of this study was to evaluate safety and efficacy of the ASEPT system. Method. This single centre, prospective study enrolled 50 patients with MPE, who were followed for as long as they were alive with a catheter. Quality of Life (QoL) was assessed before, at 2 weeks, and 6 weeks after ASEPT catheter insertion using the EORTC QLQ-C30 and LC13 questionnaires. Ease of catheter use and complications were reported by physician and community nurses. Results. 50 patients with MPE with a mean age of 64.5 ± 1.9, BDI of 2.8 ± 0.9, and ECOG score of 3.0 ± 0.7 were recruited. No immediate or long-term complications were reported during the study period. Compared to precatheter insertion, global health status (-18, p < 0.001), QLQ-C30 dyspnea (-39, p < 0.00001), and LC13 dyspnea (-11, p < 0.0005) significantly improved at 2 and 6 weeks after intervention. Provider surveys indicated favourable ease of use. Conclusion. The new ASEPT catheter offers a safe and effective option for the management of MPE.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
The impact of tunneled pleural catheters on the quality of life of patients with malignant pleural effusions.Respiration. 2013;85(1):36-42. doi: 10.1159/000342343. Epub 2012 Nov 13. Respiration. 2013. PMID: 23154202
-
Quality-of-Life assessment in malignant pleural effusion treated with indwelling pleural catheter: a prospective study.Palliat Med. 2014 Apr;28(4):326-34. doi: 10.1177/0269216314521851. Epub 2014 Feb 12. Palliat Med. 2014. PMID: 24523284
-
Successful use of central venous catheters in the management of recurrent malignant pleural effusions: one new option.Support Care Cancer. 2015 Aug;23(8):2267-71. doi: 10.1007/s00520-014-2595-3. Epub 2015 Jan 10. Support Care Cancer. 2015. PMID: 25576432
-
Use of the Pleurx Pleural Catheter for the management of malignant pleural effusions.Clin J Oncol Nurs. 2003 Jan-Feb;7(1):35-8. doi: 10.1188/03.CJON.35-38. Clin J Oncol Nurs. 2003. PMID: 12629932 Review.
-
Interventional therapies for malignant pleural effusions: the present and the future.Respirology. 2014 Aug;19(6):809-22. doi: 10.1111/resp.12328. Epub 2014 Jun 19. Respirology. 2014. PMID: 24947955 Review.
Cited by
-
Cost Comparison of Treatment Alternatives for Pleural Effusion and Ascites from a Payer Perspective: Are There Cost Savings from Indwelling Catheters?J Palliat Med. 2023 Nov;26(11):1510-1520. doi: 10.1089/jpm.2022.0592. Epub 2023 Jun 23. J Palliat Med. 2023. PMID: 37352428 Free PMC article.
-
Effect of medical thoracoscopy-guided intrapleural docetaxel therapy to manage malignant pleural effusion in patients with non-small cell lung cancer: A pilot study.Thorac Cancer. 2019 Oct;10(10):1885-1892. doi: 10.1111/1759-7714.13158. Epub 2019 Aug 6. Thorac Cancer. 2019. PMID: 31389192 Free PMC article.
References
-
- Davies H. E., Mishra E. K., Kahan B. C., et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. The Journal of the American Medical Association. 2012;307(22):2383–2389. doi: 10.1001/jama.2012.5535. - DOI - PubMed
-
- Patz E. F., Jr. Malignant pleural effusions: recent advances and ambulatory sclerotherapy. Chest. 1998;113(supplement 1):74S–77S. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

