The mito-DAMP cardiolipin blocks IL-10 production causing persistent inflammation during bacterial pneumonia
- PMID: 28074841
- PMCID: PMC5241690
- DOI: 10.1038/ncomms13944
The mito-DAMP cardiolipin blocks IL-10 production causing persistent inflammation during bacterial pneumonia
Abstract
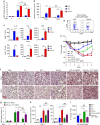
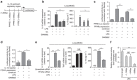
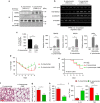
Bacterial pneumonia is a significant healthcare burden worldwide. Failure to resolve inflammation after infection precipitates lung injury and an increase in morbidity and mortality. Gram-negative bacteria are common in pneumonia and increased levels of the mito-damage-associated molecular pattern (DAMP) cardiolipin can be detected in the lungs. Here we show that mice infected with Klebsiella pneumoniae develop lung injury with accumulation of cardiolipin. Cardiolipin inhibits resolution of inflammation by suppressing production of anti-inflammatory IL-10 by lung CD11b+Ly6GintLy6CloF4/80+ cells. Cardiolipin induces PPARγ SUMOylation, which causes recruitment of a repressive NCOR/HDAC3 complex to the IL-10 promoter, but not the TNF promoter, thereby tipping the balance towards inflammation rather than resolution. Inhibition of HDAC activity by sodium butyrate enhances recruitment of acetylated histone 3 to the IL-10 promoter and increases the concentration of IL-10 in the lungs. These findings identify a mechanism of persistent inflammation during pneumonia and indicate the potential of HDAC inhibition as a therapy.
Figures





References
-
- Nathan C. & Ding A. Nonresolving inflammation. Cell 140, 871–882 (2010). - PubMed
-
- Bhattacharya J. & Matthay M. A. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu. Rev. Physiol. 75, 593–615 (2013). - PubMed
-
- Buckley C. D., Gilroy D. W., Serhan C. N., Stockinger B. & Tak P. P. The resolution of inflammation. Nat. Rev. Immunol. 13, 59–66 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

