Antiproliferative Effects of 1α-OH-vitD3 in Malignant Melanoma: Potential Therapeutic implications
- PMID: 28074906
- PMCID: PMC5225467
- DOI: 10.1038/srep40370
Antiproliferative Effects of 1α-OH-vitD3 in Malignant Melanoma: Potential Therapeutic implications
Abstract
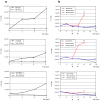
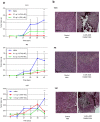
Early detection and surgery represent the mainstay of treatment for superficial melanoma, but for high risk lesions (Breslow's thickness >0.75 mm) an effective adjuvant therapy is lacking. Vitamin D insufficiency plays a relevant role in cancer biology. The biological effects of 1α hydroxycholecalciferol on experimental melanoma models were investigated. 105 melanoma patients were checked for 25-hydroxycholecalciferol (circulating vitamin D) serum levels. Human derived melanoma cell lines and in vivo xenografts were used for studying 1α-hydroxycholecalciferol-mediated biological effects on cell proliferation and tumor growth. 99 out of 105 (94%) melanoma patients had insufficient 25-hydroxycholecalciferol serum levels. Interestingly among the six with vitamin D in the normal range, five had a diagnosis of in situ/microinvasive melanoma. Treatment with 1α-hydroxycholecalciferol induced antiproliferative effects on melanoma cells in vitro and in vivo, modulating the expression of cell cycle key regulatory molecules. Cell cycle arrest in G1 or G2 phase was invariably observed in vitamin D treated melanoma cells. The antiproliferative activity induced by 1α-hydroxycholecalciferol in experimental melanoma models, together with the discovery of insufficient 25-hydroxycholecalciferol serum levels in melanoma patients, provide the rationale for using vitamin D in melanoma adjuvant therapy, alone or in association with other therapeutic options.
Figures
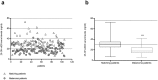
 ) and in 101 matched (for sex and age) healthy individuals (∆). (b) The area between 30–76 ng/mL, which represents 25-OH-vitD sufficiency, is marked by a gray line. Standard deviation is shown (Statistical analysis using t-test: ***p value < 0.005).
) and in 101 matched (for sex and age) healthy individuals (∆). (b) The area between 30–76 ng/mL, which represents 25-OH-vitD sufficiency, is marked by a gray line. Standard deviation is shown (Statistical analysis using t-test: ***p value < 0.005).


References
-
- Siegel R., Naishadham D. & Jemal A. Cancer statistics, 2012. CA. Cancer J. Clin. 62, 10–29 (2012). - PubMed
-
- Tsao H., Atkins M. B. & Sober A. J. Management of cutaneous melanoma. N Engl J Med 351, 998–1012 (2004). - PubMed
-
- Cummins D. L. et al. Cutaneous malignant melanoma. Mayo Clin. Proc. 81, 500–507 (2006). - PubMed
-
- Korn E. L. et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J. Clin. Oncol. 26, 527–534 (2008). - PubMed
-
- Kudchadkar R. & Sondak V. Adjuvant interferon: recommit or move on? Lancet Oncol. 12, 112–113 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

