The Helicobacter cinaedi antigen CAIP participates in atherosclerotic inflammation by promoting the differentiation of macrophages in foam cells
- PMID: 28074932
- PMCID: PMC5225449
- DOI: 10.1038/srep40515
The Helicobacter cinaedi antigen CAIP participates in atherosclerotic inflammation by promoting the differentiation of macrophages in foam cells
Abstract
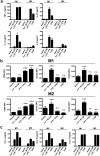
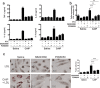
Recent studies have shown that certain specific microbial infections participate in atherosclerosis by inducing inflammation and immune reactions, but how the pathogens implicated in this pathology trigger the host responses remains unknown. In this study we show that Helicobacter cinaedi (Hc) is a human pathogen linked to atherosclerosis development since at least 27% of sera from atherosclerotic patients specifically recognize a protein of the Hc proteome, that we named Cinaedi Atherosclerosis Inflammatory Protein (CAIP) (n = 71). CAIP appears to be implicated in this pathology because atheromatous plaques isolated from atherosclerotic patients are enriched in CAIP-specific T cells (10%) which, in turn, we show to drive a Th1 inflammation, an immunopathological response typically associated to atherosclerosis. Recombinant CAIP promotes the differentiation and maintenance of the pro-inflammatory profile of human macrophages and triggers the formation of foam cells, which are a hallmark of atherosclerosis. This study identifies CAIP as a relevant factor in atherosclerosis inflammation linked to Hc infection and suggests that preventing and eradicating Hc infection could reduce the incidence of atherosclerosis.
Figures






References
-
- Mozaffarian D. et al.. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131, e29–322 (2015). - PubMed
-
- Hansson G. K. & Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 12, 204–212 (2011). - PubMed
-
- Shashkin P., Dragulev B. & Ley K. Macrophage differentiation to foam cells. Curr. Pharm. Des. 11, 3061–3072 (2005). - PubMed
-
- Hansson G. K., Robertson A.-K. L. & Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol 1, 297–329 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

