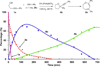
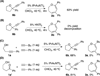
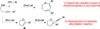Accessing alternative reaction pathways of the intermolecular condensation between homo-propargyl alcohols and terminal alkynes through divergent gold catalysis
- PMID: 28074975
- PMCID: PMC5313346
- DOI: 10.1039/c6cc09794d
Accessing alternative reaction pathways of the intermolecular condensation between homo-propargyl alcohols and terminal alkynes through divergent gold catalysis
Abstract
An intermolecular condensation of alkynols and terminal alkynes is reported. Using IPrAuNTf2, an efficient Au-catalyzed cyclization-alkynylation strategy furnishes (2-arylalkynyl) cyclic ethers in moderate to excellent yields (up to 94%). This strategy is extended to the synthesis of functionalized 2,3-dihydrooxepines via the sequential Au-catalyzed ring expansion of the cyclic ether substrates.
Figures
Similar articles
-
Theoretical Insight into the Au(I)-Catalyzed Intermolecular Condensation of Homopropargyl Alcohols with Terminal Alkynes: Reactant Stoichiometric Ratio-Controlled Chemodivergence.J Org Chem. 2019 Jan 18;84(2):579-588. doi: 10.1021/acs.joc.8b02411. Epub 2018 Nov 15. J Org Chem. 2019. PMID: 30394741
-
Gold-Catalyzed Vinyl Ether Hydroalkynylation: An Alternative Pathway for the Gold-Catalyzed Intermolecular Reaction of Alkenes and Alkynes.Org Lett. 2016 Dec 16;18(24):6336-6339. doi: 10.1021/acs.orglett.6b03228. Epub 2016 Nov 29. Org Lett. 2016. PMID: 27978688 Free PMC article.
-
Gold-Catalyzed Cyclization of Alkyne Alcohols: Regioselective Construction of Functionalized 6,6- and 6,7-Bicyclic Ethers.Chem Pharm Bull (Tokyo). 2016;64(7):845-55. doi: 10.1248/cpb.c16-00204. Chem Pharm Bull (Tokyo). 2016. PMID: 27373641
-
Azido-Alkynes in Gold(I)-Catalyzed Indole Syntheses.Chem Rec. 2021 Dec;21(12):3897-3910. doi: 10.1002/tcr.202100202. Epub 2021 Sep 8. Chem Rec. 2021. PMID: 34498385 Review.
-
Advances in gold catalyzed synthesis of quinoid heteroaryls.RSC Adv. 2024 Jul 3;14(29):21047-21064. doi: 10.1039/d4ra03368j. eCollection 2024 Jun 27. RSC Adv. 2024. PMID: 38962094 Free PMC article. Review.
Cited by
-
Hexafluoroisopropanol-Promoted Disulfidation and Diselenation of Alkyne, Alkene, and Allene.Org Lett. 2020 Jul 17;22(14):5462-5465. doi: 10.1021/acs.orglett.0c01834. Epub 2020 Jun 26. Org Lett. 2020. PMID: 32588633 Free PMC article.
-
Gold-Catalyzed Hydroalkoxylation/Povarov Reaction Cascade of Alkynols with N-Aryl Imines: Synthesis of Tetrahydroquinolines.ACS Omega. 2019 Sep 9;4(13):15754-15763. doi: 10.1021/acsomega.9b02693. eCollection 2019 Sep 24. ACS Omega. 2019. PMID: 31572879 Free PMC article.
References
-
-
For a collection of recent reviews, see: Pflasterer S, Hashmi ASK. Chem. Soc. Rev. 2016;45:1331–1367. Dorel R, Echavarren AM. Chem. Rev. 2015;115:9028. Rudolph M, Hashmi ASK. Chem. Soc. Rev. 2012;41:2448. Krause N, Winter C. Chem. Rev. 2011;111:1994. Fürstner A. Chem. Soc. Rev. 2009;38:3208.
-
-
- Chen G-Q, Fang W, Wei Y, Tang X-Y, Shi M. Chem. Sci. 2016;7:4318. - PMC - PubMed
- Fang W, Tang X-Y, Shi M. RSC Adv. 2016;6:4047.
- Tomás-Mendivil E, Toullec PY, Díez J, Conejero S, Michelet V, Cadierno V. Org. Lett. 2012;14:2520. - PubMed
- Alcaide B, Almendros P, Cembellín S, del Campo TM, Fernández I. Chem. Commun. 2013;49:1282. - PubMed
- Meiss R, Kumar K, Waldmann H. Chem. Eur. J. 2015;21:13526. - PubMed
- Miró J, Sánchez-Roselló M, González J, del Pozo C, Fustero S. Chem. Eur. J. 2015;21:5459. - PubMed
- Rao WD, Koh MJ, Kothandaraman P, Chan PWH. J. Am. Chem. Soc. 2012;134:10811. - PubMed
- Brady PB, Carreira EM. Org. Lett. 2015;17:3350. - PubMed
-
- Wei Y, Shi M. ACS Catal. 2016;6:2515.
-
- Motika SE, Wang Q, Akhmedov NG, Wojtas L, Shi X. Angew. Chem., Int. Ed. 2016;55:11582. - PubMed
- Yang Y, Qin A, Zhao K, Wang D, Shi X. Adv. Synth. Catal. 2016;358:1433.
- Wang Q, Motika SE, Akhmedov NG, Petersen JL, Shi X. Angew. Chem., Int. Ed. 2014;53:5418. - PubMed
- Duan H, Sengupta S, Petersen JL, Akhmedov NG, Shi X. J. Am. Chem. Soc. 2009;131:12100. - PubMed
-
- Hosseyni S, Wojtas L, Li M, Shi X. J. Am. Chem. Soc. 2016;138:3994. - PubMed
- Snieckus V, Frota LC. Synfacts. 2016;12:573.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources