Diagnostic Value of Serum Angiogenesis Markers in Ovarian Cancer Using Multiplex Immunoassay
- PMID: 28075407
- PMCID: PMC5297757
- DOI: 10.3390/ijms18010123
Diagnostic Value of Serum Angiogenesis Markers in Ovarian Cancer Using Multiplex Immunoassay
Abstract
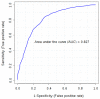
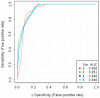
As cancer development involves pathological vessel formation, 16 angiogenesis markers were evaluated as potential ovarian cancer (OC) biomarkers. Blood samples collected from 172 patients were divided based on histopathological result: OC (n = 38), borderline ovarian tumours (n = 6), non-malignant ovarian tumours (n = 62), healthy controls (n = 50) and 16 patients were excluded. Sixteen angiogenesis markers were measured using BioPlex Pro Human Cancer Biomarker Panel 1 immunoassay. Additionally, concentrations of cancer antigen 125 (CA125) and human epididymis protein 4 (HE4) were measured in patients with adnexal masses using electrochemiluminescence immunoassay. In the comparison between OC vs. non-OC, osteopontin achieved the highest area under the curve (AUC) of 0.79 (sensitivity 69%, specificity 78%). Multimarker models based on four to six markers (basic fibroblast growth factor-FGF-basic, follistatin, hepatocyte growth factor-HGF, osteopontin, platelet-derived growth factor AB/BB-PDGF-AB/BB, leptin) demonstrated higher discriminatory ability (AUC 0.80-0.81) than a single marker (AUC 0.79). When comparing OC with benign ovarian tumours, six markers had statistically different expression (osteopontin, leptin, follistatin, PDGF-AB/BB, HGF, FGF-basic). Osteopontin was the best single angiogenesis marker (AUC 0.825, sensitivity 72%, specificity 82%). A three-marker panel consisting of osteopontin, CA125 and HE4 better discriminated the groups (AUC 0.958) than HE4 or CA125 alone (AUC 0.941 and 0.932, respectively). Osteopontin should be further investigated as a potential biomarker in OC screening and differential diagnosis of ovarian tumours. Adding osteopontin to a panel of already used biomarkers (CA125 and HE4) significantly improves differential diagnosis between malignant and benign ovarian tumours.
Keywords: angiogenesis; biomarkers; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ferlay J., Shin H., Bray F., Forman D., Mathers C., Parkin D. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. [(accessed on 1 September 2016)]. Available online: https://www.iarc.fr/en/media-centre/iarcnews/2010/globocan2008.php.
-
- Heintz A.P.M., Odicino F., Maisonneuve P., Quinn M.A., Benedet J.L., Creasman W.T., Ngan H.Y. S., Pecorelli S., Beller U. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006;95:161–192. doi: 10.1016/S0020-7292(06)60033-7. - DOI - PubMed
-
- Doubeni C.A., Doubeni A.R., Myers A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician. 2016;93:937–944. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

