Spinosad Induces Antioxidative Response and Ultrastructure Changes in Males of Red Palm Weevil Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae)
- PMID: 28076286
- PMCID: PMC5066058
- DOI: 10.1093/jisesa/iew089
Spinosad Induces Antioxidative Response and Ultrastructure Changes in Males of Red Palm Weevil Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae)
Abstract
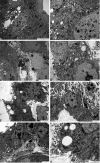
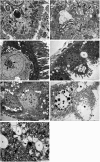
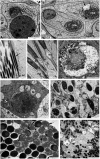
The red palm weevil, Rhynchophorus ferrugineus, is of great concern worldwide, especially in the Middle East, where dates are a strategic crop. Despite their ecological hazard, insecticides remain the most effective means of control. A bioinsecticide of bacterial origin, spinosad is effective against several pests, and its efficacy against male R. ferrugineus was assessed in the present study. The antioxidative responses of key enzymes including catalase (CAT), superoxide dismutase (SOD), and glutathione-S-transferase (GST) to spinosad were investigated in the midgut and testes, and the effects of this insecticide on the cell ultrastructure of the midgut, Malpighian tubules, and testes were also determined. The lethal concentration 50 of spinosad was measured at 58.8 ppm, and the insecticide inhibited the activities of CAT, SOD, and GST in the midgut. However, no significant changes in the activities of these enzymes were observed in the testes. Spinosad treatment resulted in concentration-dependent changes in the cellular organelles of the midgut, Malpighian tubules, and testes of R. ferrugineus, and some of these effects were similar to those exerted by other xenobiotics. However, specific changes were observed as a result of spinosad treatment, including an increase in the number and size of concretions in Malpighian tubule cells and the occasional absence of the central pair of microtubules in the axonemes of sperm tails. This study introduces spinosad for potential use as an insecticide within an integrated control program against male red palm weevils. Additionally, the study provides biochemical and ultrastructural evidence for use in the development of bioindicators.
Keywords: Malpighian tubule; Rhynchophorus ferrugineus; Spinosad; antioxidant; midgut; testis; ultrastructure.
© The Authors 2016. Published by Oxford University Press on behalf of Entomological Society of America.
Figures


References
-
- Abarikwu S. O., Duru Q. C., Chinonso O. V., Njoku R. C. 2015. Antioxidant enzymes activity, lipid peroxidation, oxidative damage in the testis and epididymis, and steroidogenesis in rats after co-exposure to atrazine and ethanol. Andrologia. 48(5): 548–557. - PubMed
-
- Abbott W. S. 1925. A method of computing effectiveness of an insecticide. J. Econ. Entomol. 18: 265–267.
-
- Abdelsalam S. A., Korayem A. M., Elsherbini E. A. M., Abdel-Aal A. A., Mohamed D. S. 2014. Photosensitizing Effects of Hematoporphyrin Dihydrochloride against the Flesh Fly Parasarcophaga argyrostoma (Diptera: Sarcophagidae). Florida Entomologist. 97: 1662–1670.
-
- Abouelghar G. E., Sakr H., Ammar H. A., Yousef A., Nassar M. 2013. Sublethal Effects of Spinosad (Tracer®) on the Cotton Leafworm (Lepidoptera: Noctuidae). J. Plant Prot. Res. 53: 275–284.
-
- Aebi H. 1984. Catalase in vitro. Methods Enzymol. 105(C): 121–126. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

