Feasibility of abbreviated cycles of immunochemotherapy for completely resected limited-stage CD20+ diffuse large B-cell lymphoma (CISL 12-09)
- PMID: 28076329
- PMCID: PMC5355104
- DOI: 10.18632/oncotarget.14531
Feasibility of abbreviated cycles of immunochemotherapy for completely resected limited-stage CD20+ diffuse large B-cell lymphoma (CISL 12-09)
Abstract
Background: The appropriate number of chemotherapy cycles for limited stage diffuse large B-cell lymphoma (DLBCL) patients without gross residual lesions after complete resection, has not been specifically questioned. We performed a multicenter, single-arm, phase 2 study to investigate the feasibility of 3 cycles of abbreviated R-CHOP chemotherapy in low-risk patients with completely resected localized CD20+ DLBCL.
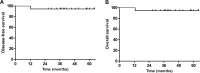
Results: Between December 2010 and May 2013, we recruited 23 patients. One was excluded due to ineligibility, and hence, 22 were included in the final analysis. The primary sites comprised the intestine (n = 15), cervical lymph nodes (n = 4), stomach (n = 1), tonsil (n = 1), and spleen (n = 1). All patients successfully completed the 3 cycles of planned R-CHOP chemotherapy. Over a median follow-up of 39.5 months (95% confidence interval, 29.9-47.1 months), both the estimated 2-year disease-free survival and overall survival rates was 95% confidence interval, 85.9-104.1%. Only one patient with an international prognostic index of 2 experienced relapse and died. The most common grade 3 or 4 toxicity condition included neutropenia (n = 8, 36.4%). Three patients experienced grade 3 febrile neutropenia, but no grade 3 or 4 non-hematologic toxicity was observed.
Materials and methods: DLBCL patients without residual lesions after resection were enrolled and R-CHOP chemotherapy was repeated at 3-week-intervals over 3 cycles. The primary endpoint was 2-year disease-free survival.
Conclusions: Three cycles of abbreviated R-CHOP immunochemotherapy is feasible for completely resected low risk localized DLBCL.
Keywords: abbreviated cycles and R-CHOP chemotherapy; complete resection; diffuse large B-cell lymphoma; limited stage.
Conflict of interest statement
DH Yoon, SY Oh, and C Suh have received research funding from Roche. None of the other authors have relevant conflicts of interest to declare.
Figures
References
-
- Persky DO, Unger JM, Spier CM, Stea B, LeBlanc M, McCarty MJ, Rimsza LM, Fisher RI, Miller TP. Phase II Study of Rituximab Plus Three Cycles of CHOP and Involved-Field Radiotherapy for Patients With Limited-Stage Aggressive B-Cell Lymphoma: Southwest Oncology Group Study 0014. J Clin Oncol. 2008;26:2258–2263. - PubMed
-
- Sehn LH, Savage K, Hoskins P, Klasa R, Shenkier T, Gascoyne RD, Voss N, Wilson D, Connors J. Limited-stage DLBCL patients with a negative FDG-PET scan following three cycles of R-CHOP have an excellent outcome following abbreviated immuno-chemotherapy alone. Ann Oncol. 2008;19:iv99–100.
-
- Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, Grogan TM, LeBlanc M, Carlin S, Chase E, Fisher RI. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med. 1998;339:21–26. - PubMed
-
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. - PubMed
-
- Divine M, Casassus P, Koscielny S, Bosq J, Sebban C, Le Maignan C, Stamattoulas A, Dupriez B, Raphael M, Pico JL, Ribrag V. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005;16:1928–1935. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials