Gene Expression Profiling in Slow-Type Calf Soleus Muscle of 30 Days Space-Flown Mice
- PMID: 28076365
- PMCID: PMC5226721
- DOI: 10.1371/journal.pone.0169314
Gene Expression Profiling in Slow-Type Calf Soleus Muscle of 30 Days Space-Flown Mice
Abstract
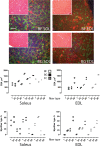
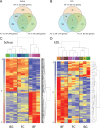
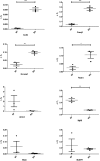
Microgravity exposure as well as chronic disuse are two main causes of skeletal muscle atrophy in animals and humans. The antigravity calf soleus is a reference postural muscle to investigate the mechanism of disuse-induced maladaptation and plasticity of human and rodent (rats or mice) skeletal musculature. Here, we report microgravity-induced global gene expression changes in space-flown mouse skeletal muscle and the identification of yet unknown disuse susceptible transcripts found in soleus (a mainly slow phenotype) but not in extensor digitorum longus (a mainly fast phenotype dorsiflexor as functional counterpart to soleus). Adult C57Bl/N6 male mice (n = 5) flew aboard a biosatellite for 30 days on orbit (BION-M1 mission, 2013), a sex and age-matched cohort were housed in standard vivarium cages (n = 5), or in a replicate flight habitat as ground control (n = 5). Next to disuse atrophy signs (reduced size and myofiber phenotype I to II type shift) as much as 680 differentially expressed genes were found in the space-flown soleus, and only 72 in extensor digitorum longus (only 24 genes in common) compared to ground controls. Altered expression of gene transcripts matched key biological processes (contractile machinery, calcium homeostasis, muscle development, cell metabolism, inflammatory and oxidative stress response). Some transcripts (Fzd9, Casq2, Kcnma1, Ppara, Myf6) were further validated by quantitative real-time PCR (qRT-PCR). Besides previous reports on other leg muscle types we put forth for the first time a complete set of microgravity susceptible gene transcripts in soleus of mice as promising new biomarkers or targets for optimization of physical countermeasures and rehabilitation protocols to overcome disuse atrophy conditions in different clinical settings, rehabilitation and spaceflight.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Fitts RH, Riley DR, Widrick JJ. Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol (1985). 2000;89(2):823–39. Epub 2000/08/05. - PubMed
-
- Baldwin KM. Effect of spaceflight on the functional, biochemical, and metabolic properties of skeletal muscle. Medicine and science in sports and exercise. 1996;28(8):983–7. Epub 1996/08/01. - PubMed
-
- Caiozzo VJ, Haddad F, Baker MJ, Herrick RE, Prietto N, Baldwin KM. Microgravity-induced transformations of myosin isoforms and contractile properties of skeletal muscle. J Appl Physiol (1985). 1996;81(1):123–32. Epub 1996/07/01. - PubMed
-
- Chopard A, Leclerc L, Pons F, Leger JJ, Marini JF. Effects of 14-day spaceflight on myosin heavy chain expression in biceps and triceps muscles of the rhesus monkey. Journal of gravitational physiology: a journal of the International Society for Gravitational Physiology. 2000;7(1):S47–9. Epub 2001/09/07. - PubMed
-
- Harrison BC, Allen DL, Girten B, Stodieck LS, Kostenuik PJ, Bateman TA, et al. Skeletal muscle adaptations to microgravity exposure in the mouse. J Appl Physiol (1985). 2003;95(6):2462–70. Epub 2003/07/29. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

