Comparative Analysis of Vertebrate Diurnal/Circadian Transcriptomes
- PMID: 28076377
- PMCID: PMC5226840
- DOI: 10.1371/journal.pone.0169923
Comparative Analysis of Vertebrate Diurnal/Circadian Transcriptomes
Abstract
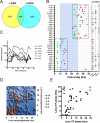
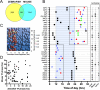
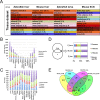
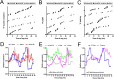
From photosynthetic bacteria to mammals, the circadian clock evolved to track diurnal rhythms and enable organisms to anticipate daily recurring changes such as temperature and light. It orchestrates a broad spectrum of physiology such as the sleep/wake and eating/fasting cycles. While we have made tremendous advances in our understanding of the molecular details of the circadian clock mechanism and how it is synchronized with the environment, we still have rudimentary knowledge regarding its connection to help regulate diurnal physiology. One potential reason is the sheer size of the output network. Diurnal/circadian transcriptomic studies are reporting that around 10% of the expressed genome is rhythmically controlled. Zebrafish is an important model system for the study of the core circadian mechanism in vertebrate. As Zebrafish share more than 70% of its genes with human, it could also be an additional model in addition to rodent for exploring the diurnal/circadian output with potential for translational relevance. Here we performed comparative diurnal/circadian transcriptome analysis with established mouse liver and other tissue datasets. First, by combining liver tissue sampling in a 48h time series, transcription profiling using oligonucleotide arrays and bioinformatics analysis, we profiled rhythmic transcripts and identified 2609 rhythmic genes. The comparative analysis revealed interesting features of the output network regarding number of rhythmic genes, proportion of tissue specific genes and the extent of transcription factor family expression. Undoubtedly, the Zebrafish model system will help identify new vertebrate outputs and their regulators and provides leads for further characterization of the diurnal cis-regulatory network.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Williams GE. Geological constraints on the Precambrian history of Earth“s rotation and the Moon”s orbit. Reviews of Geophysics. 2000.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

