Role for Egr1 in the Transcriptional Program Associated with Neuronal Differentiation of PC12 Cells
- PMID: 28076410
- PMCID: PMC5226839
- DOI: 10.1371/journal.pone.0170076
Role for Egr1 in the Transcriptional Program Associated with Neuronal Differentiation of PC12 Cells
Abstract
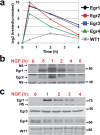
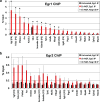
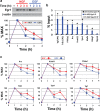
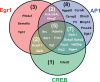
PC12 cells are a well-established model to study how differences in signal transduction duration can elicit distinct cell behaviors. Epidermal growth factor (EGF) activates transient ERK signaling in PC12 cells that lasts 30-60 min, which in turn promotes proliferation; nerve growth factor (NGF) activates more sustained ERK signaling that lasts 4-6 h, which in turns induces neuronal differentiation. Data presented here extend a previous study by Mullenbrock et al. (2011) that demonstrated that sustained ERK signaling in response to NGF induces preferential expression of a 69-member gene set compared to transient ERK signaling in response to EGF and that the transcription factors AP-1 and CREB play a major role in the preferential expression of several genes within the set. Here, we examined whether the Egr family of transcription factors also contributes to the preferential expression of the gene set in response to NGF. Our data demonstrate that NGF causes transient induction of all Egr family member transcripts, but a corresponding induction of protein was detected for only Egr1 and 2. Chromatin immunoprecipitation experiments provided clearest evidence that, after induction, Egr1 binds 12 of the 69 genes that are preferentially expressed during sustained ERK signaling. In addition, Egr1 expression and binding upstream of its target genes were both sustained in response to NGF versus EGF within the same timeframe that its targets are preferentially expressed. These data thus provide evidence that Egr1 contributes to the transcriptional program activated by sustained ERK signaling in response to NGF, specifically by contributing to the preferential expression of its target genes identified here.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proceedings of the National Academy of Sciences of the United States of America. 1976;73(7):2424–8. Epub 1976/07/01. PubMed Central PMCID: PMC430592. - PMC - PubMed
-
- Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. The Journal of biological chemistry. 1995;270(23):13585–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

