Signaling Lymphocytic Activation Molecule Family Member 7 Engagement Restores Defective Effector CD8+ T Cell Function in Systemic Lupus Erythematosus
- PMID: 28076903
- PMCID: PMC5406259
- DOI: 10.1002/art.40038
Signaling Lymphocytic Activation Molecule Family Member 7 Engagement Restores Defective Effector CD8+ T Cell Function in Systemic Lupus Erythematosus
Abstract
Objective: Effector CD8+ T cell function is impaired in systemic lupus erythematosus (SLE) and is associated with a compromised ability to fight infections. Signaling lymphocytic activation molecule family member 7 (SLAMF7) engagement has been shown to enhance natural killer cell degranulation. This study was undertaken to characterize the expression and function of SLAMF7 on CD8+ T cell subsets isolated from the peripheral blood of SLE patients and healthy subjects.
Methods: CD8+ T cell subset distribution, SLAMF7 expression, and expression of cytolytic enzymes (perforin, granzyme A [GzmA], and GzmB) on cells isolated from SLE patients and healthy controls were analyzed by flow cytometry. CD107a expression and interferon-γ (IFNγ) production in response to viral antigenic stimulation in the presence or absence of an anti-SLAMF7 antibody were assessed by flow cytometry. Antiviral cytotoxic activity in response to SLAMF7 engagement was determined using a flow cytometry-based assay.
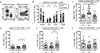
Results: The distribution of CD8+ T cell subsets was altered in the peripheral blood of SLE patients, with a decreased effector cell subpopulation. Memory CD8+ T cells from SLE patients displayed decreased amounts of SLAMF7, a surface receptor that characterizes effector CD8+ T cells. Ligation of SLAMF7 increased CD8+ T cell degranulation capacity and the percentage of IFNγ-producing cells in response to antigen challenge in SLE patients and healthy controls. Moreover, SLAMF7 engagement promoted cytotoxic lysis of target cells in response to stimulation with viral antigens.
Conclusion: CD8+ T cell activation in response to viral antigens is defective in SLE patients. Activation of SLAMF7 through a specific monoclonal antibody restores CD8+ T cell antiviral effector function to normal levels and thus represents a potential therapeutic option in SLE.
© 2017, American College of Rheumatology.
Figures


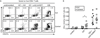

References
-
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–2121. - PubMed
-
- Stohl W. Impaired polyclonal T cell cytolytic activity. A possible risk factor for systemic lupus erythematosus. Arthritis Rheum. 1995;38(4):506–516. - PubMed
-
- Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. - PubMed
-
- Peng SL, et al. Perforin protects against autoimmunity in lupus-prone mice. J Immunol. 1998;160(2):652–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

