Clinical, biochemical and molecular characteristics of Filipino patients with mucopolysaccharidosis type II - Hunter syndrome
- PMID: 28077157
- PMCID: PMC5225557
- DOI: 10.1186/s13023-016-0558-0
Clinical, biochemical and molecular characteristics of Filipino patients with mucopolysaccharidosis type II - Hunter syndrome
Abstract
Background: Mucopolysaccharidosis type II, an X-linked recessive disorder is the most common lysosomal storage disease detected among Filipinos. This is a case series involving 23 male Filipino patients confirmed to have Hunter syndrome. The clinical and biochemical characteristics were obtained and mutation testing of the IDS gene was done on the probands and their female relatives.
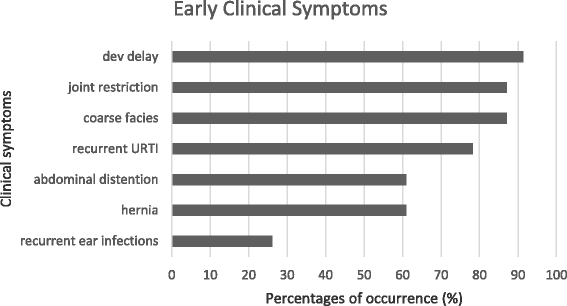
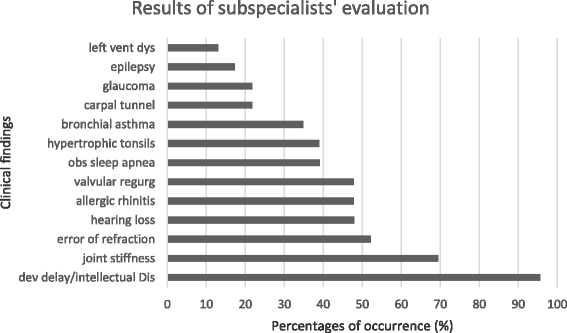
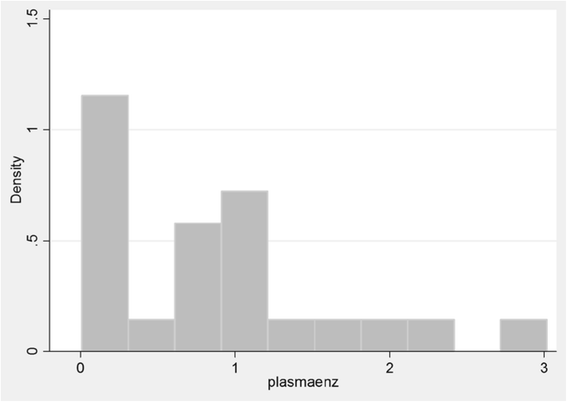
Results: The mean age of the patients was 11.28 (SD 4.10) years with an average symptom onset at 1.2 (SD 1.4) years. The mean age at biochemical diagnosis was 8 (SD 3.2) years. The early clinical characteristics were developmental delay, joint stiffness, coarse facies, recurrent respiratory tract infections, abdominal distention and hernia. Majority of the patients had joint contractures, severe intellectual disability, error of refraction, hearing loss and valvular regurgitation on subspecialists' evaluation. The mean GAG concentration was 506.5 mg (SD 191.3)/grams creatinine while the mean plasma iduronate-2-sulfatase activity was 0.86 (SD 0.79) nmol/mg plasma/4 h. Fourteen (14) mutations were found: 6 missense (42.9%), 4 nonsense (28.6%), 2 frameshift (14.3%), 1 exon skipping at the cDNA level (7.1%), and 1 gross insertion (7.1%). Six (6) novel mutations were observed (43%): p.C422F, p.P86Rfs*44, p.Q121*, p.L209Wfs*4, p.T409R, and c.1461_1462insN[710].
Conclusion: The age at diagnosis in this series was much delayed and majority of the patients presented with severe neurologic impairment. The results of the biochemical tests did not contribute to the phenotypic classification of patients. The effects of the mutations were consistent with the severe phenotype seen in the majority of the patients.
Keywords: Glycosaminoglycans; Hunter syndrome; Iduronate-2-sulfatase gene; Lysosomal storage disease; Mucopolysaccharidosis type II.
Figures




References
-
- Neufeld E, Muenzer J. The Mucopolysaccharidoses. In: Scriver C, Sly W, Childs B, Beaudet A, Valle D, Kinzler K, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3421–3452.
-
- Lin S, Chang J, Lee-Chen G, Lin D, Lin H, Chuang C. Detection of Hunter syndrome (mucopolysaccharidosis type II) in Taiwanese: biochemical and linkage studies of the iduronate-2-sulfatase gene defects in MPS II patients and carriers. Clin Chim Acta. 2006;369:29–34. doi: 10.1016/j.cca.2006.01.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

